Understanding the impacts of dual methionine oxidations in complementarity-determining regions on the structure of monoclonal antibodies
- PMID: 39487762
- PMCID: PMC11540082
- DOI: 10.1080/19420862.2024.2422898
Understanding the impacts of dual methionine oxidations in complementarity-determining regions on the structure of monoclonal antibodies
Abstract
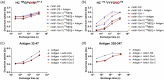
Methionine oxidation can substantially alter the structure and functionality of monoclonal antibodies (mAbs), especially when it occurs in the complementarity-determining regions (CDRs). It is imperative to fully understand the effects of methionine oxidation because these modifications can affect the binding affinity, stability, and immunogenicity of mAbs. Moreover, the presence of multiple methionines in close proximity within the amino acid sequence increases the complexity of accurate characterization, and sophisticated analytical methods are required to detect these modifications. In this study, we used hydrogen deuterium exchange mass spectrometry (HDX-MS) and homology modeling to investigate the effects of dual methionine oxidations (heavy chain (HC) Met111 and Met115) within a single CDR on the structure of a mAb. Our findings reveal that the solvent-accessible methionine (HC Met111) is more prone to oxidation, but such a modification does not result in conformational changes in the mAb. In contrast, the methionine (HC Met115) at the VH-VL interface, when subjected to different oxidative stresses, can undergo oxidation with selective stereochemistry. This can lead to predominant formation of either the S- or R-form of methionine sulfoxide diastereomer, each of which can induce distinct local conformational changes. A mechanism is proposed to elucidate these observations in this particular antibody. Furthermore, binding assays confirm that both CDR methionine oxidations do not compromise antigen binding, which alleviates concerns about potential loss of therapeutic efficacy.
Keywords: Antibody higher order structure; Antigen binding; Complementarity determining region; HDX-MS; Methionine oxidation; Stereochemistry; homology modeling.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
