Three-dimensional dynamics of mesothelin-targeted CAR.CIK lymphocytes against ovarian cancer peritoneal carcinomatosis
- PMID: 39487859
- PMCID: PMC11531451
- DOI: 10.1007/s00262-024-03860-w
Three-dimensional dynamics of mesothelin-targeted CAR.CIK lymphocytes against ovarian cancer peritoneal carcinomatosis
Abstract
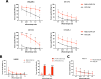
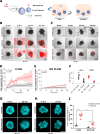
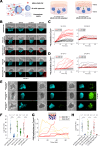
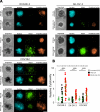
Intraperitoneal cellular immunotherapy with CAR-redirected lymphocytes is an intriguing approach to target peritoneal carcinomatosis (PC) from ovarian cancer (OC), which is currently evaluated in clinical trials. PC displays a composite structure with floating tumor cells within ascites and solid-like masses invading the peritoneum. Therefore, a comprehensive experimental model is crucial to optimize CAR-cell therapies in such a peculiar environment. Here, we explored the activity of cytokine-induced killer lymphocytes (CIK), redirected by CAR against mesothelin (MSLN-CAR.CIK), within reductionistic 3D models resembling the structural complexity of both liquid and solid components of PC. MSLN-CAR.CIK effectively killed and were functionally efficient against OC targets. In a "floating-like" 3D context with floating OC spheroids, both tumor localization and killing by MSLN-CAR.CIK were significantly boosted by fluid flow. In a "solid-like" context, MSLN-CAR.CIK were recruited through the extracellular matrix on embedded tumor aggregates, with variable kinetics depending on the effector-target distance. Furthermore, MSLN-CAR.CIK penetrated the inner levels of OC spheroids exerting effective tumor killing. Our findings provide currently unknown therapeutically relevant information on intraperitoneal approaches with CAR.CIK, supporting further developments and improvements for clinical studies in the context of locoregional cell therapy approaches for patients with PC from OC.
Keywords: 3D models; Cellular immunotherapy; Chimeric antigen receptor; Ovarian cancer; Peritoneal carcinomatosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures

References
-
- Cortés-Guiral D, Hübner M, Alyami M, Bhatt A, Ceelen W, Glehen O et al (2021) Primary and metastatic peritoneal surface malignancies. Nat Rev Dis Primers 7(1):91 - PubMed
MeSH terms
Substances
Grants and funding
- 2023-2024/Italian Ministry of Health, Ricerca Corrente
- 2023-2024/Italian Ministry of Health, Ricerca Corrente
- 2023-2024/Italian Ministry of Health, Ricerca Corrente
- 2023-2024/Italian Ministry of Health, Ricerca Corrente
- 2023-2024/Italian Ministry of Health, Ricerca Corrente
- 2023-2024/Italian Ministry of Health, Ricerca Corrente
- 2023-2024/Italian Ministry of Health, Ricerca Corrente
- 23211/Fondazione AIRC per la ricerca sul cancro ETS
- 25040/Fondazione AIRC per la ricerca sul cancro ETS
- 20259/Fondazione AIRC per la ricerca sul cancro ETS
- 2019/Ricerca Locale, Università degli Studi di Torino
- 2022/Ricerca Locale, Università degli Studi di Torino
- 2022/Fondazione CRT
- RCR-2019-23669115/CAR-T Grant
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

