Prognostic and predictive factors for the efficacy and safety of trastuzumab deruxtecan in HER2-positive gastric or gastroesophageal junction cancer
- PMID: 39487862
- PMCID: PMC11706866
- DOI: 10.1007/s10120-024-01560-z
Prognostic and predictive factors for the efficacy and safety of trastuzumab deruxtecan in HER2-positive gastric or gastroesophageal junction cancer
Abstract
Background: Trastuzumab deruxtecan (T-DXd) is an antibody-drug conjugate targeting HER2-positive gastric cancer or gastroesophageal junction cancer (GC/GEJC). Although effective, T-DXd has notable toxicities, including interstitial lung disease (ILD). This study evaluated the efficacy, safety, and prognostic factors associated with T-DXd for GC/GEJC.
Methods: A retrospective observational study was conducted at our institution by reviewing medical records of patients treated with T-DXd until September 2023. Eligible patients had unresectable advanced or recurrent GC/GEJC, HER2 status of IHC 3 + or IHC 2 + /ISH-positive, and prior treatment with trastuzumab-containing regimen.
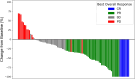
Results: Among the 101 patients analyzed, the initial T-DXd dose was 6.4 mg/kg in 77 patients and 5.4 mg/kg in 24 patients. The objective response rate was 54.3%, with a median PFS of 5.4 months and a median OS of 11.4 months. The significant prognostic factors for shorter PFS and OS included ECOG PS ≥ 1, presence of primary lesion, and peritoneal metastasis but not the initial T-DXd dose. ILD occurred in 14.9% of patients. Notably, higher T-DXd dose and smaller tumor burden were associated with a higher incidence of ILD.
Conclusions: Several factors were associated with prognosis after T-DXd treatment in patients with GC/GEJC. Tumor burden is a potential risk factor for T-DXd-related ILD. Further studies are needed to optimize dosing based on tumor burden and to improve the therapeutic index.
Keywords: Gastric cancer; Interstitial lung disease; Optimize dosing; Trastuzumab deruxtecan; Tumor burden.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: A. Jubashi have no conflict of interest. I. Nakayama has received research funding from Ono, MSD, and CHUGAI PHARMACEUTICAL CO., LTD. outside the submitted work. S. Koganemaru has received research funding from Eisai Inc., Amgen, Bristol-Myers Squibb, MSD, Daiichi Sankyo, AbbVie and Incyte outside the submitted work. Y. Matsubara has received honoraria from Taiho, Takeda, Elli Lilly Japan, MSD, and Bristol-Myers Squibb outside the submitted work. S. Mishima has received honoraria from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., and Eli Lilly Co., Ltd. outside the submitted work. D. Kotani has received honoraria from Takeda, Chugai, Lilly, MSD, Ono, Taiho, Bristol-Myers Squibb, Daiichi Sankyo, Pfizer, Eisai, and Merck biopharma and research funding from Ono, MSD, Novartis, Servier, Janssen, IQVIA, Syneos Health, Cimic, and Cimicshiftzero outside the submitted work. A. Kawazoe has received honoraria from Daiichi Sankyo, Lilly, Ono, Taiho, Bristol-Myers Squibb, and Merck Serono biopharma and research funding from Ono, MSD, Taiho, Bayer, Sumitomo Dainippon and AstraZeneca outside the submitted work. T. Hashimoto has received honoraria from CytoGen, Inc. and Takata Pharmaceutical outside the submitted work. Y. Nakamura plays an advisory role at Guardant Health Pte Ltd., Natera, Inc., Roche Ltd., Seagen, Inc., Premo Partners, Inc., Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., Exact Sciences Corporation, and Gilead Sciences, Inc.; is included in the speakers’ bureau from Guardant Health Pte Ltd., MSD K.K., Eisai Co., Ltd., Zeria Pharmaceutical Co., Ltd., Miyarisan Pharmaceutical Co., Ltd., Merck Biopharma Co., Ltd., CareNet, Inc., Hisamitsu Pharmaceutical Co., Inc., Taiho Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Chugai Pharmaceutical Co., Ltd., Becton, Dickinson and Company, and Guardant Health Japan Corp; and has received research funding from Seagen, Inc., Genomedia Inc., Guardant Health AMEA, Inc., Guardant Health, Inc., Tempus Labs, Inc., Roche Diagnostics K.K., Daiichi Sankyo Co., Ltd., and Chugai Pharmaceutical Co., Ltd. outside the submitted work. Y. Kuboki has received personal fees for advisory roles from Taiho, Takeda, and Boehringer Ingelheim; honoraria from Taiho, Ono, Bayer, and Sanofi; and institutional grants or contracts with Taiho, Takeda, Ono, AbbVie, AstraZeneca, Boehringer Ingelheim, Incyte, Amgen, Chugai, GlaxoSmithKline, Genmab, Astellas, and Daiichi Sankyo outside the submitted work. H. Bando has received research fund from Ono Pharmaceutical and honoraria from Ono Pharmaceutical, Taiho Pharmaceutical, and Eli Lilly Japan outside the submitted work. T. Kojima has received honoraria as an invited speaker and for manuscript writing from Bristol-Myers Squibb, Ono Pharmaceutical Co., Ltd., Covidien Japan, Inc., MSD K.K., TAIHO PHARMACEUTICAL CO., LTD., and Oncolys BioPharma Inc.; research funding from Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb, BeiGene Ltd., EPS Corporation., MSD K.K., Amgen Inc., SHIONOGI & Co., Ltd., and CHUGAI PHARMACEUTICAL CO., LTD. outside the submitted work. T. Yoshino has received honoraria from Chugai Pharmaceutical, Takeda Pharmaceutical, Merck Biopharma, Bayer Yakuhin, Ono Pharmaceutical, and MSD K.K; consulting fee from Sumitomo Corp.; and research funding from Amgen, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, FALCO biosystems, Genomedia, Medical & Biological Laboratories, Merus N.V., Molecular Health GmbH, MSD, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Pfizer Japan, Roche Diagnostics, Sanofi, Sysmex, Taiho Pharmaceutical, and Takeda Pharmaceutical outside the submitted work. K. Nakao has received honoraria from AstraZeneca outside the submitted work. K. Shitara has received personal fees for advisory roles from Lilly, Bristol-Myers Squibb, Takeda, Pfizer, Ono Pharmaceutical, Merck Pharmaceutical, Taiho Pharmaceutical, Novartis, AbbVie, GlaxoSmithKline, Daiichi Sankyo, Amgen, Boehringer Ingelheim, and Janssen; honoraria (lecture fee) from Takeda and Bristol-Myers Squibb; and research funding from Astellas, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Chugai, Merck Pharmaceutical, Medi Science, Eisai, and Amgen outside the submitted work. All remaining authors have no competing interest. Informed consent: All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later versions. Informed consent to be included in the study or the equivalent was obtained from all patients.
Figures


References
-
- Van Cutsem E, di Bartolomeo M, Smyth E, Chau I, Park H, Siena S, et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 2023;24:744–56. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

