Transcutaneous Auricular Vagus Nerve Stimulation for Visually Induced Motion Sickness: An eLORETA Study
- PMID: 39487878
- PMCID: PMC11531436
- DOI: 10.1007/s10548-024-01088-6
Transcutaneous Auricular Vagus Nerve Stimulation for Visually Induced Motion Sickness: An eLORETA Study
Abstract
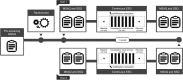
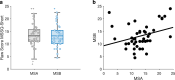
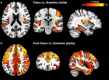
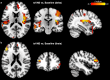
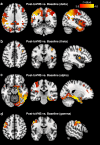
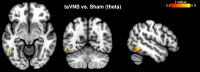
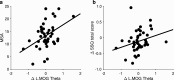
Transcutaneous auricular vagus nerve stimulation (taVNS), a non-invasive form of electrical brain stimulation, has shown potent therapeutic potential for a wide spectrum of conditions. How taVNS influences the characterization of motion sickness - a long mysterious syndrome with a polysymptomatic onset - remains unclear. Here, to examine taVNS-induced effects on brain function in response to motion-induced nausea, 64-channel electroencephalography (EEG) recordings from 42 healthy participants were analyzed; collected during nauseogenic visual stimulation concurrent with taVNS administration, in a crossover randomized sham-controlled study. Cortical neuronal generators were estimated from the obtained EEG using exact low-resolution brain electromagnetic tomography (eLORETA). While both sham and taVNS increased insula activation during electrical stimulation, compared to baseline, taVNS additionally augmented middle frontal gyrus neuronal activity. Following taVNS, brain regions including the supramarginal, parahippocampal, and precentral gyri were activated. Contrasting sham, taVNS markedly increased activity in the middle occipital gyrus during stimulation. A repeated-measures ANOVA showed that taVNS reduced motion sickness symptoms. This reduction in symptoms correlated with taVNS-induced neural activation. Our findings provide new insights into taVNS-induced brain changes, during and after nauseogenic stimuli exposure, including accompanying behavioral response. Together, these findings suggest that taVNS has promise as an effective neurostimulation tool for motion sickness management.
Keywords: Electroencephalography; Motion sickness; Source localization; Transcutaneous auricular vagus nerve stimulation; eLORETA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no Conflict of interest.
Figures
References
-
- Angelaki DE, Cullen KE (2008) Vestibular System: The Many Facets of a Multimodal Sense. Annu. Rev. Neurosci. 31(1):125–150 - PubMed
-
- Adelhöfer N, Gohil K, Passow S, Beste C, Li S-C (2019) Lateral prefrontal anodal transcranial direct current stimulation augments resolution of auditory perceptual-attentional conflicts. NeuroImage 199:217–227 - PubMed
-
- Astafiev SV, Stanley CM, Shulman GL, Corbetta M (2004) Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nature Neuroscience 7(5):542–548 - PubMed
-
- Bos JE, Bles W (2004) Motion sickness induced by optokinetic drums. Aviation, Space, and Environmental Medicine 75(2):172–174 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

