Therapeutic drug monitoring in children and adolescents with schizophrenia-spectrum, affective, behavioural, tic and other psychiatric disorders treated with aripiprazole: results of the TDM-VIGIL pharmacovigilance study
- PMID: 39487894
- PMCID: PMC11785646
- DOI: 10.1007/s00702-024-02819-6
Therapeutic drug monitoring in children and adolescents with schizophrenia-spectrum, affective, behavioural, tic and other psychiatric disorders treated with aripiprazole: results of the TDM-VIGIL pharmacovigilance study
Abstract
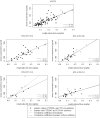
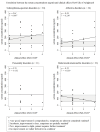
Aripiprazole is approved for various severe mental disorders in adults and adolescents. However, off-label prescribing is common, especially in children and adolescents (youth) in whom aripiprazole therapeutic serum level reference ranges are lacking for any disorders. The aim of the study was to evaluate the relationship between aripiprazole dose and serum concentrations and provide further knowledge on the use of aripiprazole in order to improve drug safety and effectiveness in the treatment of minors. The clinical course of youth treated with aripiprazole in the multicentre pharmacovigilance study TDM-VIGIL was systematically followed and serum concentrations measured. Sex, age, weight and comedications were analysed to identify possible effect modifiers. A preliminary therapeutic reference range was estimated for youth with schizophrenia-spectrum disorders, affective disorders and behavioural/emotional/tic disorders coded as treatment responders based on a Clinical-Global Impressions-Improvement (CGI-I) score of much or very much improved. In 93 youth (mean age = 15.2 ± 2.6, range = 7.4-18.2 years, females = 53%, CGI-Severity = 4.4 ± 1.1, responders = 64%), a positive, moderate correlation between the weight-normalized daily dose (WNDD) and aripiprazole serum concentration (=0.791, p < 0.0001) was found. The WNDD and co-medications that interact with CYP2D6 and CYP3A4 affected aripiprazole serum levels, explaining 64% of the variance. In patients within the preliminary therapeutic ranges determined by interquartile ranges (IQRs), slightly better outcomes and fewer adverse drug reactions were found versus patients within preliminary therapeutic ranges determined by the mean ± SD. The preliminary reference range for paediatric patients with schizophrenia-spectrum disorders calculated by the IQR showed an identical lower threshold (100-230 ng/ml) compared to adult schizophrenia-spectrum disorders patients (100-350 ng/ml). The preliminary therapeutic ranges for patients with affective disorders was: 60-160 ng/ml and for patients with behavioural/tic disorders 60-140 ng/ml. The therapeutic reference ranges for aripiprazole in youth estimated via the 25th and 75th IQRs may result in more clinically relevant therapeutic windows. Further studies need to confirm these results, especially in patients with affective and behavioural/tic disorder diagnoses.
Keywords: Affective disorder; Aripiprazole; Children and adolescents; Schizophrenia; Serum concentration; Therapeutic drug monitoring.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: KE, MR, RT and PP received grant research support from BfArM. MR currently receives a research grant from Kids-Safe, Innovation Committee of the German Federal Joint Committee (G-BA grant number 01NVF16021). PP receives grant research support from the German Federal Ministry of Education and Research (BMBF) and was involved in clinical trials from Servier and Lundbeck; he received an advisor honorarium from Boehringer Ingelheim and speaker’s honoraria from Shire, Infectopharm and Gerot Lannach. CUC has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Biogen, Boehringer-Ingelheim, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Denovo, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Neurelis, Newron, Noven, Novo Nordisk, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Seqirus, SK Life Science, Sunovion, Sun Pharma, Supernus, Takeda, Teva, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Compass Pathways, Denovo, Lundbeck, Relmada, Reviva, Rovi, Sage, Supernus, Tolmar and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and is also a stock option holder of Cardio Diagnostics, Mindpax, LB Pharma, PsiloSterics and Quantic. TB received personal fees from serving as an advisor or consultant to from eye level, Infectopharm, Lundbeck, Medice, Neurim Pharmaceuticals, Oberberg GmbH, Roche and Takeda; receiving conference attendance support, conference support, or speaking fees from or speaker’s fee by Janssen, Medice and Takeda; and receiving royalties from Hogrefe, Kohlhammer, CIP-Medien, and Oxford University Press. S.W.has received in the last 5 years royalties from Thieme, Hogrefe, Kohlhammer, Springer, Beltz. In 2023 she will receive speakers honorary from Takeda and Medice. Her work was supported in the last years by the Swiss National Science Foundation (SNF), diff. EU FP7s, HSM Hochspezialisierte Medizin of the Kanton Zurich, Switzerland, Bfarm Germany, ZInEP, Hartmann Müller Stiftung, Olga Mayenfisch, Gertrud Thalmann, Vontobel, Unicentia, Erika Schwarz, Heuberg Fonds, National Government of Health (BAG), Gesundheitsförderung Schweiz and Horizon Europe. Outside professional activities and interests are declared under the link of the University of Zurich www.uzh.ch/prof/ssl-dir/interessenbindungen/client/web/ MKoe receives royalties from publishing companies as an author of books. He served as PI or CI in clinical trials of Lundbeck, Pascoe and Janssen-Cilag. He served as scientific advisor for Janssen. The present work is unrelated to the above grants and relationships. The other authors (JC, HC, KSM, RT, SF, CW, AK, KR, UM, PH, SU, MSC, HR, GA, WB, TH, MKae, CR, GS, MG, and CF) declare no conflict of interest.
Figures
References
-
- Bachmann CJ, Rieger-Gies A, Heinzel-Gutenbrunner M, Hiemke C, Remschmidt H, Theisen FM (2008) Large variability of aripiprazole and dehydroaripiprazole serum concentrations in adolescent patients with schizophrenia. Ther Drug Monit 30(4):462–466 - PubMed
-
- Baumann P, Hiemke C, Ulrich S, Eckermann G, Gaertner I, Gerlach M, Kuss H-J, Laux G, Müller-Oerlinghausen B, Rao ML, Riederer P, Zernig G (2004) The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry 37(6):243–265 - PubMed
-
- Conley RR, Kelly DL (2007) Drug-drug interactions associated with second-generation antipsychotics: considerations for clinicians and patients. Psychopharmacol Bull 40(1):77–97 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

