TMEM16F regulates pathologic α-synuclein secretion and spread in cellular and mouse models of Parkinson's disease
- PMID: 39487963
- PMCID: PMC11822650
- DOI: 10.1111/acel.14387
TMEM16F regulates pathologic α-synuclein secretion and spread in cellular and mouse models of Parkinson's disease
Abstract
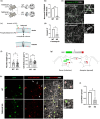
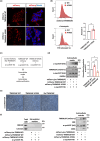
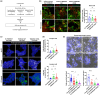
One of the main hallmarks of Parkinson's disease (PD) pathology is the spread of the aggregate-prone protein α-synuclein (α-syn), which can be detected in the plasma and cerebrospinal fluid of patients as well as in the extracellular environment of neuronal cells. The secreted α-syn can exhibit "prion-like" behavior and transmission to naïve cells can promote conformational changes and pathology. The precise role of plasma membrane proteins in the pathologic process of α-syn is yet to be fully resolved. The TMEM16 family of lipid scramblases and ion channels has been recently associated with cancer and infectious diseases but is less known for its role in aging-related diseases. To elucidate the role of TMEM16F in α-syn spread, we transduced neurons derived from TMEM16F knockout mice with a reporter system that enables the distinction between donor and recipient neurons of pathologic α-synA53T. We found that the spread of α-synA53T was reduced in neurons derived from TMEM16F-knockout mice. These findings were recapitulated in vivo in a mouse model of PD, where attenuated α-synA53T spread was observed when TMEM16F was ablated. Moreover, we identified a single nucleotide polymorphism in TMEM16F of Ashkenazi Jewish PD patients resulting in a missense Ala703Ser mutation with enhanced lipid scramblase activity. This mutation is associated with altered regulation of α-synA53T extracellular secretion in cellular models of PD. Our study highlights TMEM16F as a novel regulator of α-syn spread and as a potential therapeutic target in synucleinopathies.
Keywords: aggregate‐prone proteins; aging‐related diseases; extracellular secretion; lipid translocases; proteostasis.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors report no competing interests.
Figures



References
-
- Anderson, J. P. , Walker, D. E. , Goldstein, J. M. , de Laat, R. , Banducci, K. , Caccavello, R. J. , Barbour, R. , Huang, J. , Kling, K. , Lee, M. , Diep, L. , Keim, P. S. , Shen, X. , Chataway, T. , Schlossmacher, M. G. , Seubert, P. , Schenk, D. , Sinha, S. , Gai, W. P. , & Chilcote, T. J. (2006). Phosphorylation of Ser‐129 is the dominant pathological modification of alpha‐synuclein in familial and sporadic Lewy body disease. The Journal of Biological Chemistry, 281, 29739–29752. - PubMed
-
- Arend, R. C. , Londono‐Joshi, A. I. , Gangrade, A. , Katre, A. A. , Kurpad, C. , Li, Y. , Samant, R. S. , Li, P. K. , Landen, C. N. , Yang, E. S. , Hidalgo, B. , Alvarez, R. D. , Straughn, J. M. , Forero, A. , & Buchsbaum, D. J. (2016). Niclosamide and its analogs are potent inhibitors of Wnt/beta‐catenin, mTOR and STAT3 signaling in ovarian cancer. Oncotarget, 7, 86803–86815. - PMC - PubMed
-
- Braak, H. , Ghebremedhin, E. , Rub, U. , Bratzke, H. , & Del Tredici, K. (2004). Stages in the development of Parkinson's disease‐related pathology. Cell and Tissue Research, 318, 121–134. - PubMed
-
- Braga, L. , Ali, H. , Secco, I. , Chiavacci, E. , Neves, G. , Goldhill, D. , Penn, R. , Jimenez‐Guardeno, J. M. , Ortega‐Prieto, A. M. , Bussani, R. , Cannatà, A. , Rizzari, G. , Collesi, C. , Schneider, E. , Arosio, D. , Shah, A. M. , Barclay, W. S. , Malim, M. H. , Burrone, J. , & Giacca, M. (2021). Drugs that inhibit TMEM16 proteins block SARS‐CoV‐2 spike‐induced syncytia. Nature, 594, 88–93. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

