Phase II trial evaluating the long-term efficacy and peripheral sensory neuropathy of capecitabine plus oxaliplatin (XELOX) as adjuvant therapy in Japanese patients with operated stage III colon cancer
- PMID: 39488510
- PMCID: PMC11531565
- DOI: 10.1038/s41598-024-73222-0
Phase II trial evaluating the long-term efficacy and peripheral sensory neuropathy of capecitabine plus oxaliplatin (XELOX) as adjuvant therapy in Japanese patients with operated stage III colon cancer
Abstract
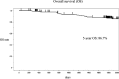
Adjuvant oxaliplatin plus capecitabine (XELOX) therapy is recommended for patients with curatively resected colon cancer. However, prospective data on its practical application in Japanese patients are limited. Therefore, we aimed to conduct a long-term clinical evaluation of the efficacy and safety of adjuvant XELOX in patients with curatively resected stage III colon cancer (MCSCO-1024). This prospective, multi-center, open-label, single-arm, phase II study enrolled patients with curatively resected stage III colon cancer. The treatment protocol consisted of a 120-minute intravenous infusion of oxaliplatin (130 mg/m2) on day 1 and two divided doses oral capecitabine (2000 mg/m2/day) for 14 days in a 3-week cycle, totaling eight cycles (24 weeks). The primary endpoint was 3-year disease-free survival (DFS), and the secondary endpoints were 5-year overall survival and long-term prognosis of peripheral sensory neuropathy. A total of 196 patients were enrolled between November 2011 and August 2014 (34 months). The 3-year DFS rate was 73%, and the 5-year overall survival rate was 87%. The overall incidence of peripheral sensory neuropathy was 17%, with a 1% rate of grade 3 neuropathy after 5 years. Adjuvant XELOX demonstrated utility and safety in the clinical management of Japanese patients with stage III colon cancer.
Keywords: Adjuvant chemotherapy; Japanese; Peripheral sensory neuropathy; XELOX.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Chiu, J. et al. Efficacy and tolerability of adjuvant oral capecitabine plus intravenous oxaliplatin (XELOX) in Asian patients with colorectal cancer: 4-year analysis. Asian Pac. J. Cancer Prev.14, 6585–6590 (2013). - PubMed
-
- Danno, K. et al. Interim analysis of a phase II trial evaluating the safety and efficacy of capecitabine plus oxaliplatin (XELOX) as adjuvant therapy in Japanese patients with operated stage III colon cancer. Cancer Chemother. Pharmacol.80, 777–785 (2017). - PubMed
-
- André, T. et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol.27, 3109–3116 (2009). - PubMed
-
- Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal Carcinoma 2nd edn (English) (Kanehara Shuppan, 2009).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources