Effectiveness of BNT162b2 XBB vaccine in the US Veterans Affairs Healthcare System
- PMID: 39488521
- PMCID: PMC11531596
- DOI: 10.1038/s41467-024-53842-w
Effectiveness of BNT162b2 XBB vaccine in the US Veterans Affairs Healthcare System
Abstract
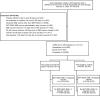
Data evaluating effectiveness of XBB.1.5-adapted vaccines against JN.1-related endpoints are scarce. This nationwide test-negative case-control study within the US Veterans Affairs Healthcare System aims to estimate vaccine effectiveness (VE) of BNT162b2 XBB.1.5-adapted vaccine compared to not receiving an XBB vaccine of any kind against COVID-19 hospitalization, emergency department or urgent care visits (ED/UC), and outpatient visits. Between September 25, 2023 and January 31, 2024, effectiveness was 24-35% during a period of JN.1 predominance and 50-61% during XBB predominance across all outcomes. VE within 60 days of vaccination during the likely JN.1 period was 32% (95% confidence interval 3-52%) against hospitalization, 41% (23-54%) against ED/UC visits, and 31% (1-52%) against outpatient visits. Corresponding VE during the likely XBB period was 62% (44-74%), 52% (37-63%), and 50% (25-66%) by setting, respectively. Here, we show the importance of strain match to maximize the public health impact of COVID-19 vaccination.
© 2024. The Author(s).
Conflict of interest statement
Haley J. Appaneal has received research funding from Pfizer. Aisling R. Caffrey has received research funding from AbbVie, Merck, and Pfizer. Vrishali V. Lopes has no competing interest to declare. Kerry L. LaPlante has received research funding from AbbVie, Merck, and Pfizer and has been an advisor for Ferring Pharmaceuticals, AbbVie, and Seres Therapeutics. Laura Puzniak, Evan J. Zasowski, Luis Jodar, and John M. McLaughlin are employees and shareholders of Pfizer Inc.
Figures

References
-
- World Health Organization (WHO). Coronavirus (COVID-19) Dashboard. Accessed 21 Feb 2024, https://covid19.who.int/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

