Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles: A focus on inflammatory bowel disease
- PMID: 39488745
- PMCID: PMC11531661
- DOI: 10.1002/ctm2.70075
Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles: A focus on inflammatory bowel disease
Abstract
Background: Mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) have emerged as key regulators of intercellular communication, orchestrating essential biological processes by delivering bioactive cargoes to target cells. Available evidence suggests that MSC-EVs can mimic the functions of their parental cells, exhibiting immunomodulatory, pro-regenerative, anti-apoptotic, and antifibrotic properties. Consequently, MSC-EVs represent a cell-free therapeutic option for patients with inflammatory bowel disease (IBD), overcoming the limitations associated with cell replacement therapy, including their non-immunogenic nature, lower risk of tumourigenicity, cargo specificity and ease of manipulation and storage.
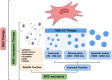
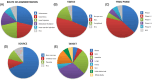
Main topics covered: This review aims to provide a comprehensive examination of the therapeutic efficacy of MSC-EVs in IBD, with a focus on their mechanisms of action and potential impact on treatment outcomes. We examine the advantages of MSC-EVs over traditional therapies, discuss methods for their isolation and characterisation, and present mechanistic insights into their therapeutic effects through transcriptomic, proteomic and lipidomic analyses of MSC-EV cargoes. We also discuss available preclinical studies demonstrating that MSC-EVs reduce inflammation, promote tissue repair and restore intestinal homeostasis in IBD models, and compare these findings with those of clinical trials.
Conclusions: Finally, we highlight the potential of MSC-EVs as a novel therapy for IBD and identify challenges and opportunities associated with their translation into clinical practice.
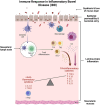
Highlights: The source of mesenchymal stem cells (MSCs) strongly influences the composition and function of MSC-derived extracellular vesicles (EVs), affecting their therapeutic potential. Adipose-derived MSC-EVs, known for their immunoregulatory properties and ease of isolation, show promise as a treatment for inflammatory bowel disease (IBD). MicroRNAs are consistently present in MSC-EVs across cell types and are involved in pathways that are dysregulated in IBD, making them potential therapeutic agents. For example, miR-let-7a is associated with inhibition of apoptosis, miR-100 supports cell survival, miR-125b helps suppress pro-inflammatory cytokines and miR-20 promotes anti-inflammatory M2 macrophage polarisation. Preclinical studies in IBD models have shown that MSC-EVs reduce intestinal inflammation by suppressing pro-inflammatory mediators (e.g., TNF-α, IL-1β, IL-6) and increasing anti-inflammatory factors (e.g., IL-4, IL-10). They also promote mucosal healing and strengthen the integrity of the gut barrier, suggesting their potential to address IBD pathology.
Keywords: Crohn's disease; exosomes; nanomedicine; ulcerative colitis.
© 2024 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures


References
-
- Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1‐10. - PubMed
-
- Le Berre C, Ananthakrishnan AN, Danese S, Singh S, Peyrin‐Biroulet L. Ulcerative colitis and Crohn's disease have similar burden and goals for treatment. Clin Gastroenterol Hepatol. 2020;18:14‐23. - PubMed
-
- Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458‐466. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
