A Systematic Literature Review of Modelling Approaches to Evaluate the Cost Effectiveness of PET/CT for Therapy Response Monitoring in Oncology
- PMID: 39488797
- PMCID: PMC11782410
- DOI: 10.1007/s40273-024-01447-y
A Systematic Literature Review of Modelling Approaches to Evaluate the Cost Effectiveness of PET/CT for Therapy Response Monitoring in Oncology
Abstract
Background and objective: This systematic literature review addresses model-based cost-effectiveness studies for therapy response monitoring with positron emission tomography (PET) generally combined with low-dose computed tomography (CT) for various cancer types. Given the known heterogeneity in therapy response events, studies should consider patient-level modelling rather than cohort-based modelling because of its flexibility in handling these events and the time to events. This review aims to identify the modelling methods used and includes a systematic assessment of the assumptions made in the current literature.
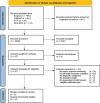
Methods: This study was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement. Information sources included electronic bibliographic databases, reference lists of review articles and contact with experts in the fields of nuclear medicine, health technology assessment and health economics. Eligibility criteria included peer-reviewed scientific publications and published grey literature. Literature searches, screening and critical appraisal were conducted by two reviewers independently. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) were used to assess the methodological quality. The Bias in Economic Evaluation (ECOBIAS) checklist was used to determine the risk of bias in the included publications.
Results: The search results included 2959 publications. The number of publications included for data extraction and synthesis was ten, representing eight unique studies. These studies addressed patients with lymphoma, advanced head and neck cancers, brain tumours, non-small cell lung cancer and cervical cancer. All studies addressed response to chemotherapy. No study evaluated response to immunotherapy. Most studies positioned PET/CT as an add-on modality and one study positioned PET/CT as a replacement for conventional imaging (X-ray and contrast-enhanced CT). Three studies reported decision-tree structures, four studies reported cohort-level state-transition models and one study reported a partitioned survival model. No patient-level models were reported. The simulation horizons adopted ranged from 1 year to lifetime. Most studies reported a probabilistic analysis, whereas two studies reported a deterministic analysis only. Two studies conducted a value of information analysis. Multiple studies did not adequately discuss model-specific aspects of bias. Most importantly and regularly observed were a high risk of structural assumptions bias, limited simulation horizon bias and wrong model bias.
Conclusions: Model-based cost-effectiveness analysis for therapy response monitoring with PET/CT was based on cohorts of patients instead of individual patients in the current literature. Therefore, the heterogeneity in therapy response events was commonly not addressed appropriately. Further research should include more advanced and patient-level modelling approaches to accurately represent the complex context of clinical practice and, therefore, to be meaningful to support decision making.
Registration: This review is registered in PROSPERO, the international prospective register of systematic reviews funded by the National Institute for Health Research, with CRD42023402581.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Funding: No funds, grants or other support were received during the preparation of this article. Conflicts of interest/competing interests: Sietse van Mossel, Rafael Emilio de Feria Cardet, Lioe-Fee de Geus-Oei, Dennis Vriens, Hendrik Koffijberg and Sopany Saing have no relevant financial or non-financial interests to disclose. Ethics approval: Not applicable. Consent to participate: Not applicable. Consent for publication: Not applicable. Availability of data and material: The datasets generated and/or analysed during the current study are available in the supplementary information. Code availability: Not applicable. Authors’ contributions: All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by SvM and RdFC. The first draft of the manuscript was written by SvM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Figures
References
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1. 1). Eur J Cancer. 2009;45:228–47. - PubMed
-
- Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. - PubMed
-
- Lopci E, Hicks RJ, Dimitrakopoulou-Strauss A, Dercle L, Iravani A, Seban RD, et al. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur J Nucl Med Mol Imaging. 2022;49:2323–41. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

