TRIM47 is a prognostic biomarker for gallbladder cancer and promotes tumor progression through regulating K63-linked ubiquitination of PARP1
- PMID: 39489093
- PMCID: PMC11567951
- DOI: 10.1016/j.tranon.2024.102164
TRIM47 is a prognostic biomarker for gallbladder cancer and promotes tumor progression through regulating K63-linked ubiquitination of PARP1
Abstract
Background: Gallbladder cancer (GBC) is one of the most lethal malignancies worldwide with an extremely poor prognosis. Previous studies have suggested that tripartite motif containing 47 (TRIM47) is involved in the progression of numerous cancers. However, the molecular mechanism and function of TRIM47 in GBC remain unclear.
Methods: The clinical significance of TRIM47 was evaluated using immunohistochemistry. Functional assays were performed in vitro and in vivo to determine the role of TRIM47 in GBC. Mass spectrometric analysis, western blotting, and immunoprecipitation assays were performed to investigate the molecular mechanisms involved.
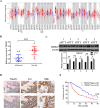
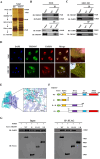
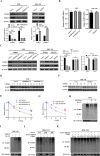
Results: In this study, TRIM47 was upregulated in GBC tissues and associated with shorter overall survival rates and TRIM47 was involved in GBC cell proliferation, migration, and apoptosis. Mechanistically, TRIM47 interacts with PARP1 and mediates the K63-linked polyubiquitination of PARP1, thereby stabilizing its expression. Furthermore, TRIM47 activated the AKT signaling pathway via PARP1.
Conclusion: The present study revealed that TRIM47 contributes to the progression of GBC and is therefore an important biomarker for predicting the prognosis of GBC and for therapeutic intervention.
Keywords: Gallbladder cancer; PARP1; Prognosis; TRIM47; Ubiquitination.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. - PubMed
-
- Vuthaluru S., Sharma P., Chowdhury S., Are C. Global epidemiological trends and variations in the burden of gallbladder cancer. J. Surg. Oncol. 2023;128(6):980–988. - PubMed
-
- Lv T.R., Hu H.J., Liu F., Ma W.J., Jin Y.W., Li F.Y. The significance of peri-neural invasion in patients with gallbladder carcinoma after curative surgery: a 10 year experience in China. Updates Surg. 2023;75(5):1123–1133. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

