Telaglenastat as an alternative to cisplatin as a radiosensitizer in the treatment of head and neck squamous cell carcinoma
- PMID: 39489210
- PMCID: PMC11583984
- DOI: 10.1016/j.canlet.2024.217320
Telaglenastat as an alternative to cisplatin as a radiosensitizer in the treatment of head and neck squamous cell carcinoma
Abstract
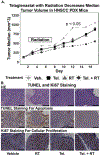
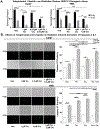
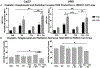
The efficacy of radiation treatment (RT) of head and neck squamous cell carcinoma (HNSCC) is limited by radioresistance and the toxicity of FDA approved radiosensitizers. In extension to our previous research where we demonstrated that telaglenastat (CB839) increased efficacy of RT in in vitro and in vivo HNSCC models, here, we examine the radiosensitizing effects of telaglenastat in comparison to cisplatin's, as cisplatin is currently the standard of care for concurrent therapy. Combination of telaglenastat with RT reduced tumor volume in a HNSCC patient derived xenograft mouse model. The efficacy of telaglenastat with RT in reducing cell survival and increasing apoptosis was similar if not greater than that of cisplatin with RT in Cal27 and HN5 HNSCC cells. The addition of telaglenastat increased reactive oxygen species and reduced the antioxidant glutathione in both Cal27 and HN5 cells. Reverse Phase Protein Array analyses revealed alterations in cell death and DNA damage response proteins. This study provides the scientific underpinnings for the use of telaglenastat as a radiosensitizer in the treatment of HNSCC either as an alternative to cisplatin or in cisplatin-ineligible patients.
Keywords: Cisplatin; Glutaminase inhibition; Head and neck squamous cell carcinoma; Radiosensitizer; Telaglenastat.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

