Patterns of loco-regional progression and patient outcomes after definitive-dose radiation therapy for anaplastic thyroid cancer
- PMID: 39489425
- PMCID: PMC11720968
- DOI: 10.1016/j.radonc.2024.110602
Patterns of loco-regional progression and patient outcomes after definitive-dose radiation therapy for anaplastic thyroid cancer
Abstract
Background: The aim of this study is to characterize the patterns of loco-regional progression (LRP) and outcomes after definitive-dose intensity modulated radiation therapy (IMRT) for anaplastic thyroid cancer (ATC) with macroscopic neck disease at the time of IMRT.
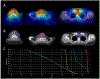
Methods: Disease/treatment characteristics and outcomes for patients with unresected or incompletely resected ATC who received IMRT (≥45 Gy) were retrospectively reviewed. For those with LRP after IMRT, progressive/recurrent gross tumor volumes (rGTV) were contoured on diagnostic CTs and co-registered with initial planning CTs using deformable image registration. rGTVs were classified based on established spatial/dosimetric criteria.
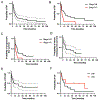
Results: Forty patients treated between 2010-2020 formed the cohort. Median IMRT dose was 66 Gy (45-70 Gy); altered fractionation (AF) was used in 24 (60 %). All received concurrent chemotherapy. In addition to areas of gross disease, target volumes (TVs) commonly included: central compartment/upper mediastinum (levels VI/VII), neck levels II-V in an involved, and levels III-IV in an uninvolved lateral neck. Median overall survival was 7.1 m. Median progression free survival was 7.4 m for patients with locoregional disease and 1.8 m for patients with distant metastasis at the time of IMRT. Twenty-one patients (53 %) developed LRP at median of 10.9 m; freedom from LRP at 3 m and 12 m was 71 % (95 %CI 58-87 %) and 47 % (95 %CI 32-68 %). Forty-one individual rGTVs were identified and most occurred within the high dose (HD) TVs: Type A/central HD (n = 29, 71 %) and B/peripheral HD (n = 3, 7 %).
Conclusions: Despite an intensive treatment schedule, including AF and concurrent chemotherapy, classic radio-resistant and rapid Type A failures predominated; isolated extraneous dose failures were rare. While these findings support the IMRT and TV delineation strategies described herein, they highlight the importance of identifying novel strategies to further improve LRC for patients with unresectable disease without targetable mutations for contemporary neo-adjuvant strategies.
Keywords: Anaplastic thyroid cancer; Definitive intensity modulated radiation therapy; Patterns of failure.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: MZ has grant funding from Eli Lilly and Merck to MD Anderson Cancer Center for ongoing phase II clinical trials. RF: Consulting/advisory role in the past 2 years:Coherus Bioscience, Eisai Medical Research, Elevar Therapeutics, Prelude Therapeutics, Regeneron Pharmaceuticals, Remix Therapeutics, Sanofi-aventis, Answers in CME, Labcorp Drug Development. Research funding (institution):ISA Therapeutics, Merck, Ayala Pharmaceuticals, Viracta, Gilead, Seagen, Prelude Therapeutics in the past 2 years. MEC: Advisory boards/consulting: Bayer, Exelixis, Lilly, Novartis. Clinical trial funding Merck, Genentech. CDF received/receives related funding and salary support from: National Institutes of Health (NIH) National Cancer Institute (NCI) and National Institute of Dental and Craniofacial Research (NIDCR) Administrative Supplements to Support Collaborations to Improve AIML-Readiness of NIH-Supported Data (R01CA257814-02S3; R01DE028290-04S2); NIH National Institute of Biomedical Imaging and Bioengineering (NIBIB) Research Education Programs for Residents and Clinical Fellows Grant (R25EB025787); NIH/NCI Cancer Center Support Grant (CCSG) (P30CA016672); Patient-Centered Outcomes Research Institute (PCS-1609-36195; sub-award from Princess Margaret Hospital). Dr. Fuller receives grant and infrastructure support from MD Anderson Cancer Center via the Charles and Daneen Stiefel Center for Head and Neck Cancer Oropharyngeal Cancer Research Program. Dr. Fuller has received NIH sub-award support from Oncospace, Inc. (R43CA254559, PI Lakshminarayanan) under a Small Business Innovation Research Grant Applications grant. Dr. Fuller has received direct industry grant/in-kind support, honoraria, and travel funding from Elekta AB. Dr. Fuller has served as a consulting capacity for Varian/Siemens Healthineers, Philips Medical Systems, and Oncospace, Inc. RD received research support from AstaZeneca, Merck, Eisai, Exellixis and serves in a cosulting role with Bayer, Novartis, Exelixis. NB: Honoraria: Eisai, Exelixis, Consulting or Advisory Role: Loxo, Eisai, Research Funding: GlaxoSmithKline/Novartis.
Figures

References
-
- Chintakuntlawar AV, Foote RL, Kasperbauer JL, Bible KC. Diagnosis and management of anaplastic thyroid cancer. Endocrinology and Metabolism Clinics of North America 2019. Mar;48(1):269–84. - PubMed
-
- Jonker PKC, Turchini J, Kruijff S, Lin JF, Gill AJ, Eade T, et al. Multimodality treatment improves locoregional control, progression-free and overall survival in patients with anaplastic thyroid cancer: a retrospective cohort study comparing oncological outcomes and morbidity between multimodality treatment and limited treatment. Annals of Surgical Oncology 2021. Nov;28(12):7520–30. - PubMed
-
- Prasongsook N, Kumar A, Chintakuntlawar AV, Foote RL, Kasperbauer J, Molina J, et al. Survival in response to multimodal therapy in anaplastic thyroid cancer. The Journal of Clinical Endocrinology and Metabolism 2017. Dec 1;102(12):4506–14. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

