Reporting of late-onset immune-related adverse events with immune checkpoint inhibitors in VigiBase
- PMID: 39489542
- PMCID: PMC11535709
- DOI: 10.1136/jitc-2024-009902
Reporting of late-onset immune-related adverse events with immune checkpoint inhibitors in VigiBase
Abstract
Background: To date, evidence on late-onset immune-related adverse events (irAEs) with immune checkpoint inhibitors (ICIs) is limited to a small number of clinical cases. This study aimed to identify drug- and patient-related characteristics potentially associated with the reporting of late-onset irAEs with ICIs in VigiBase, the WHO global database of individual case safety reports (ICSRs).
Methods: Observational study comparing deduplicated ICSRs with ICIs reporting late-onset irAEs (occurred >90 days after ICI discontinuation) versus ICSRs with ICIs not reporting late-onset irAEs, collected in VigiBase from 2011 to December 31, 2022. Logistic regression was used to model the relationship between drug-related and patient-related characteristics of ICSRs and the reporting of late-onset irAEs. Significance was determined for variables with the lower bound of the 95% CI of the reporting OR (ROR) higher than 1 and a p value <0.05.
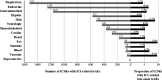
Results: The study population consisted of 6006 ICSRs with ICI-related irAEs (4574, 76.2%, originated from Europe; 3900, 64.9%, involved males; median patient age was 67 years, IQR 59-74 years). Of these, 344 (5.7%) ICSRs reported a total of 388 late-onset irAEs, among which the most frequent were thyroiditis (n=45), pneumonitis (n=37), interstitial lung disease (n=25), hepatitis (n=23) and vitiligo (n=19). Median time to onset since ICI discontinuation was 167 days (IQR 115-294 days), with negligible proportion (3.2%) of co-reported antineoplastic agents during the discontinuation period. Logistic regression models showed disproportionate reporting of late-onset irAEs with ICI combination therapy (ROR 2.33, 95% CI 1.19 to 4.57), reporting of multiple irAEs (ROR 3.96, 95% CI 2.85 to 5.52), reporting of cutaneous irAEs (ROR 1.83, 95% CI 1.24 to 2.71), and melanoma (ROR 1.47, 95% CI 1.04 to 2.06).
Conclusions: This global pharmacovigilance study provides the largest case series of late-onset irAEs with ICIs to date and identifies characteristics of ICSRs associated with disproportionate reporting. Dedicated prospective observational studies focused on long-term sequelae, quality of life and survival of patients developing late-onset irAEs with ICIs should be planned to confirm whether these reporting characteristics are predictors of actual occurrence. Furthermore, translational research should be encouraged to clarify the molecular mechanisms underlying late-onset irAE development.
Keywords: Immune Checkpoint Inhibitor; Immune related adverse event - irAE.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
