First-in-human, phase 1 dose-escalation and dose-expansion study of a RET inhibitor SY-5007 in patients with advanced RET-altered solid tumors
- PMID: 39489747
- PMCID: PMC11532403
- DOI: 10.1038/s41392-024-02006-9
First-in-human, phase 1 dose-escalation and dose-expansion study of a RET inhibitor SY-5007 in patients with advanced RET-altered solid tumors
Abstract
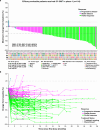
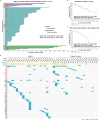
Oncogenic RET alteration is an important, tissue-agnostic therapeutic target across diverse cancers. We conducted a first-in-human phase 1 study on SY-5007, a potent and selective RET inhibitor, in patients with RET-altered solid tumors. Primary endpoints were safety, maximum tolerated dose (MTD), and recommended phase 2 dose (RP2D). Secondary endpoints included pharmacokinetics and preliminary anti-tumor activity. A total of 122 patients were enrolled (17 in dose-escalation phase and 105 in dose-expansion phase), including 91 with non-small cell lung cancer, 23 with medullary thyroid cancer, 7 with papillary thyroid cancer and 1 with gastric cancer. Treatment-related adverse events (TRAEs) were reported in 96.7% of patients, with the most common grade ≥ 3 TRAEs being hypertension (22.1%), diarrhea (16.4%), hypertriglyceridemia (6.6%), and neutropenia (6.6%). The exposure to SY-5007 was dose proportional. Among the 116 efficacy-evaluable patients, the overall objective response rate (ORR) was 57.8%, with 70.0% in treatment-naïve patients and 51.3% in previously treated patients. The median progression-free survival (PFS) was 21.1 months. Efficacy was observed regardless of tumor types and previous therapies. Biomarker analysis of 61 patients with circulating tumor DNA (ctDNA)-detectable RET alterations showed an ORR of 57.4% and median PFS of 13.8 months. Rapid ctDNA clearance of RET alteration correlated with faster responses and improved outcomes. In relapsed patients, off-target induced resistance was observed in 57.1% (12/21), with no on-target RET alterations identified. In conclusion, SY-5007 was well-tolerated and showed promising efficacy in patients with RET-altered solid tumors. Serial ctDNA monitoring may unveil treatment response and potential resistance mechanisms (NCT05278364).
© 2024. The Author(s).
Conflict of interest statement
Y.S. and X.L. are employees of Shouyao Holdings (Beijing) Co., Ltd, Beijing, China. All other authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical

