Design, synthesis, biological evaluation and computational studies of 4-Aminopiperidine-3, 4-dihyroquinazoline-2-uracil derivatives as promising antidiabetic agents
- PMID: 39489787
- PMCID: PMC11532418
- DOI: 10.1038/s41598-024-77481-9
Design, synthesis, biological evaluation and computational studies of 4-Aminopiperidine-3, 4-dihyroquinazoline-2-uracil derivatives as promising antidiabetic agents
Abstract
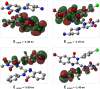
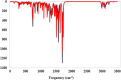
A novel series of 4-aminopiperidin-3,4-dihyroquinazoline-2-uracil derivatives (9a-9 L) were logically designed and synthesized as potent DPP4 inhibitors as antidiabetic agents. Chemical structure of all new compounds were confirmed by different spectroscopic methods. The designed compounds were evaluated using a MAK 203 kit as DPP4 inhibitors in comparison with Sitagliptin. The biological evaluation revealed that compound 9i bearing chloro substitution on phenyl moiety of 6-bromo quinazoline ring had promising inhibitory activity with IC50 = 9.25 ± 0.57 µM. The toxicity test of all compounds confirmed safety profile of them. Kinetic studies showed that compound 9i exhibited a competitive-type inhibition with a Ki value of 12.01 µM. Computational approach supported the rationality of our design strategy, as 9i represented appropriate binding interactions with the active sites of DPP4 target. MD simulation outputs validated the stability of ligand 9i at DPP4 active site. Also, Density functional theory (DFT) including HOMO-LUMO energies, ESP map, thermochemical parameters, and theoretical IR spectrum was employed to study the reactivity descriptors of 9i and 9a as the most and least potent compounds respectively. Based on the DFT study, compound 9i was softer and, as a result, more reactive than 9a. Taken together, our results showed the potential of 4-aminopiperidin-3,4-dihyroquinazoline-2-uracil derivatives as promising candidates for developing some novel DPP4 inhibitors for managing of type 2 diabetes.
Keywords: Aminopiperidine; Antidiabetic; Dihyroquinazoline; Pyrimidine.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






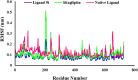
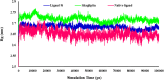
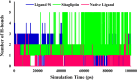


References
-
- Deshmukh, C. D., Jain, A. & Nahata, B. Diabetes mellitus: a review. Int. J. Pure Appl. Biosci.3 (3), 224–230 (2015).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

