Molecular dynamics simulations reveal concentration-dependent blockage of graphene quantum dots to water channel protein openings
- PMID: 39489799
- PMCID: PMC11532551
- DOI: 10.1038/s41598-024-77592-3
Molecular dynamics simulations reveal concentration-dependent blockage of graphene quantum dots to water channel protein openings
Abstract
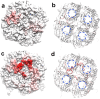
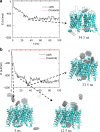
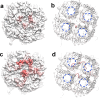
Graphene quantum dots (GQDs) have attracted significant attention across various scientific research areas due to their exceptional properties. However, studies on the potential toxicity of GQDs have yielded conflicting results. Therefore, a comprehensive evaluation of the toxicity profile of GQDs is essential for a thorough understanding of their biosafety. In this work, employing a molecular dynamics (MD) simulation approach, we investigate the interactions between GQDs and graphene oxide quantum dots (GOQDs) with the AQP1 water channel protein, aiming to explore the potential biological influence of GQDs/GOQDs. Our MD simulation results reveal that GQDs can adsorb to the loop region around the openings of AQP1 water channels, resulting in the blockage of these channels and potential toxicity. Interestingly, this blockage is concentration-dependent, with higher GQD concentrations leading to a greater likelihood of blockage. Additionally, GOQDs show a lower probability of blocking the openings of AQP1 water channels compared to GQDs, due to the hydrophobicity of the loop regions around the openings, which ultimately leads to lower interaction energy. Therefore, these findings provide new insights into the potential adverse impact of GQDs on AQP1 water channels through the blockage of their openings, offering valuable molecular insights into the toxicity profile of GQD nanomaterials.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Kroto, H. W., Heath, J. R., Obrien, S. C., Curl, R. F. & Smalley, R. E. C60: Buckminsterfullerene. Nature318(6042), 162–163 (1985).
-
- Novoselov, K. S. et al. Electric Field Effect in Atomically Thin Carbon films. Science306(5696), 666–669 (2004). - PubMed
-
- Iijima, S. Helical microtubes of graphitic carbon. Nature354(6348), 56–58 (1991).
-
- Das, A. et al. Monitoring dopants by Raman scattering in an electrochemically top-gated graphene transistor. Nat. Nanotechnol3(4), 210–215 (2008). - PubMed
-
- Fang, A., Kroenlein, K., Riccardi, D. & Smolyanitsky, A. Highly mechanosensitive Ion channels from graphene-embedded crown ethers. Nat. Mater.18(1), 76–81 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

