Manipulating the EphB4-ephrinB2 axis to reduce metastasis in HNSCC
- PMID: 39489818
- PMCID: PMC11725500
- DOI: 10.1038/s41388-024-03208-9
Manipulating the EphB4-ephrinB2 axis to reduce metastasis in HNSCC
Abstract
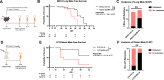
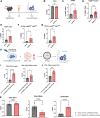
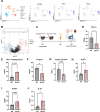
The EphB4-ephrinB2 signaling axis has been heavily implicated in metastasis across numerous cancer types. Our emerging understanding of the dichotomous roles that EphB4 and ephrinB2 play in head and neck squamous cell carcinoma (HNSCC) poses a significant challenge to rational drug design. We find that EphB4 knockdown in cancer cells enhances metastasis in preclinical HNSCC models by augmenting immunosuppressive cells like T regulatory cells (Tregs) within the tumor microenvironment. EphB4 inhibition in cancer cells also amplifies their ability to metastasize through increased expression of genes associated with hallmark pathways of metastasis along with classical and non-classical epithelial-mesenchymal transition. In contrast, vascular ephrinB2 knockout coupled with radiation therapy (RT) enhances anti-tumor immunity, reduces Treg accumulation into the tumor, and decreases metastasis. Notably, targeting the EphB4-ephrinB2 signaling axis with the engineered ligands ephrinB2-Fc-His and Fc-TNYL-RAW-GS reduces local tumor growth and distant metastasis in a preclinical model of HNSCC. Our data suggests that targeted inhibition of vascular ephrinB2 while avoiding inhibition of EphB4 in cancer cells could be a promising strategy to mitigate HNSCC metastasis.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: Dr. Karam receives clinical funding from Genentech that does not relate to this work. She receives clinical trial funding from AstraZeneca which is not related to this manuscript. She also receives preclinical research funding from Roche and Amgen, neither one of which is related to this manuscript. The remaining authors declare no competing interests. Ethics approval and consent to participate: All mice were handled and euthanized consistent with the ethics guidelines and conditions set and overseen by the University of Colorado, Anschutz Medical Campus Animal Care and Use Committee. The study has been approved by the Institutional Animal Care and Use Committee (Protocol number: 250). This study did not use any human subjects or identifiable images from human research patients.
Figures




Update of
-
Manipulating the EphB4-ephrinB2 axis to reduce metastasis in HNSCC.bioRxiv [Preprint]. 2024 Jul 23:2024.07.21.604518. doi: 10.1101/2024.07.21.604518. bioRxiv. 2024. Update in: Oncogene. 2025 Feb;44(3):130-146. doi: 10.1038/s41388-024-03208-9. PMID: 39091728 Free PMC article. Updated. Preprint.
References
-
- Duprez F, Berwouts D, De Neve W, Bonte K, Boterberg T, Deron P, et al. Distant metastases in head and neck cancer. Head Neck. 2017;39:1733–43. 10.1002/hed.24687 - PubMed
-
- Fuereder T. Essential news of current guidelines: head and neck squamous cell carcinoma. memo - Mag Eur Med Oncol. 2022;15:278–81. 10.1007/s12254-022-00842-5
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA262794/CA/NCI NIH HHS/United States
- R01CA28465 (SDK)/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P50 CA261605/CA/NCI NIH HHS/United States
- R01 DE028529/DE/NIDCR NIH HHS/United States
- P30 DK116073/DK/NIDDK NIH HHS/United States
- R01 DE028282/DE/NIDCR NIH HHS/United States
- P50CA261605-01 (SDK)/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- V Foundation (SDK/V Foundation for Cancer Research (V Foundation)
- R01 CA284651/CA/NCI NIH HHS/United States
- P30 DK063720/DK/NIDDK NIH HHS/United States
- F31 DE033887-01/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R01DE028529 (SDK)/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- F31 DE029997/DE/NIDCR NIH HHS/United States
- F31 DE033887/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

