Cancer and treatment specific incidence rates of immune-related adverse events induced by immune checkpoint inhibitors: a systematic review
- PMID: 39489880
- PMCID: PMC11723908
- DOI: 10.1038/s41416-024-02887-1
Cancer and treatment specific incidence rates of immune-related adverse events induced by immune checkpoint inhibitors: a systematic review
Erratum in
-
Correction: Cancer and treatment specific incidence rates of immune-related adverse events induced by immune checkpoint inhibitors: a systematic review.Br J Cancer. 2025 Jan;132(1):137. doi: 10.1038/s41416-024-02920-3. Br J Cancer. 2025. PMID: 39658607 Free PMC article. No abstract available.
Abstract
Background: Immune-related adverse events (irAE) induced by immune checkpoint inhibitors (ICI) are a treatment-limiting barrier. There are few large-scale studies that estimate irAE prevalence. This paper presents a systematic review that reports the prevalence of irAE by cancer type and ICI.
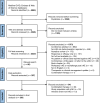
Methods: A systematic review was undertaken in MEDLINE OVID, EMBASE and Web of Science databases from 2017-2021. A total of 293 studies were identified for analysis and, of these, event rate was calculated for 272 studies, which involved 58,291 patients with irAE among 305,879 total patients on ICI. Event rate was calculated by irAE and ICI type.
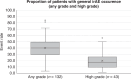
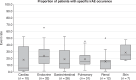
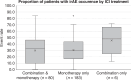
Results: Mean event rate for general irAE occurrence across any grade was 40.0% (37.3-42.7%) and high grade was 19.7% (15.8-23.7%). Mean event rates for six specific types of irAE are reported. Mean event rate for ICI monotherapy was 30.5% (28.1-32.9%), 45.7% (29.6-61.7%) for ICI combination therapy, and 30.0% (25.3-34.6%) for both ICI monotherapy and combination therapy.
Conclusion: This systematic review characterises irAE prevalence across current research that examines irAE risk factors across cancers and ICI. The findings confirms that irAE occurrence is very common in the real-world setting, both high grade and irAE across any grade.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
Comment in
-
Commentary to: Cancer and treatment specific incidence rates of immune related adverse events induced by immune checkpoint inhibitors: a systematic review.Br J Cancer. 2025 Jan;132(1):49-50. doi: 10.1038/s41416-024-02933-y. Epub 2024 Dec 16. Br J Cancer. 2025. PMID: 39681618 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

