Reversion of metabolic dysfunction-associated steatohepatitis by skeletal muscle-directed FGF21 gene therapy
- PMID: 39489916
- PMCID: PMC11638876
- DOI: 10.1016/j.ymthe.2024.10.023
Reversion of metabolic dysfunction-associated steatohepatitis by skeletal muscle-directed FGF21 gene therapy
Abstract
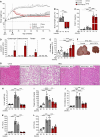
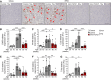
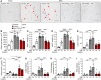
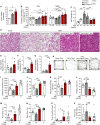
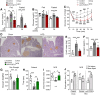
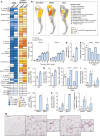
The highly prevalent metabolic dysfunction-associated steatohepatitis (MASH) is associated with liver steatosis, inflammation, and hepatocyte injury, which can lead to fibrosis and may progress to hepatocellular carcinoma and death. New treatment modalities such as gene therapy may be transformative for MASH patients. Here, we describe that one-time intramuscular administration of adeno-associated viral vectors of serotype 1 (AAV1) encoding native fibroblast growth factor 21 (FGF21), a key metabolic regulator, resulted in sustained increased circulating levels of the factor, which mediated long-term (>1 year) MASH and hepatic fibrosis reversion and halted development of liver tumors in obese male and female mouse models. AAV1-FGF21 treatment also counteracted obesity, adiposity, and insulin resistance, which are significant drivers of MASH. Scale-up to large animals successfully resulted in safe skeletal muscle biodistribution and biological activity in key metabolic tissues. Moreover, as a step toward the clinic, circulating FGF21 levels were characterized in obese, insulin-resistant and MASH patients. Overall, these results underscore the potential of the muscle-directed AAV1-FGF21 gene therapy to treat MASH and support its clinical translation.
Keywords: adeno-associated viral vectors; fibroblast growth factor 21; gene therapy; insulin resistance; metabolic dysfunction-associated steatohepatitis; metabolic dysfunction-associated steatotic liver disease; obesity; type 2 diabetes.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests V.J., V. Sacristan, C.J., I.E., I.G., A.R., and F.B. are co-inventors on patent applications for the use of AAV vectors for the treatment of metabolic diseases. F.B. is member of the scientific advisory board of Kriya Therapeutics.
Figures


References
-
- Dufour J.-F., Scherer R., Balp M.-M., McKenna S.J., Janssens N., Lopez P., Pedrosa M. The global epidemiology of nonalcoholic steatohepatitis (NASH) and associated risk factors–A targeted literature review. Endocr. Metab. Sci. 2021;3
-
- Rinella M.E., Lazarus J.V., Ratziu V., Francque S.M., Sanyal A.J., Kanwal F., Romero D., Abdelmalek M.F., Anstee Q.M., Arab J.P., et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023;79:1542–1556. - PubMed
-
- Hagström H., Nasr P., Ekstedt M., Hammar U., Stål P., Hultcrantz R., Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017;67:1265–1273. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

