Heterogeneity of the human immune response to malaria infection and vaccination driven by latent cytomegalovirus infection
- PMID: 39490199
- PMCID: PMC11576503
- DOI: 10.1016/j.ebiom.2024.105419
Heterogeneity of the human immune response to malaria infection and vaccination driven by latent cytomegalovirus infection
Abstract
Background: Human immune responses to infection and vaccination are heterogenous, driven by multiple factors including genetics, environmental exposures and personal infection histories. For malaria caused by Plasmodium falciparum parasites, host factors that impact on humoral immunity are poorly understood.
Methods: We investigated the role of latent cytomegalovirus (CMV) on the host immune response to malaria using samples obtained from individuals in previously conducted Phase 1 trials of blood stage P. falciparum Controlled Human Malaria Infection (CHMI) and in a MSP1 vaccine clinical trial. Induced antibody and functions of antibodies, as well as CD4 T cell responses were quantified.
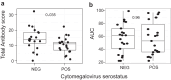
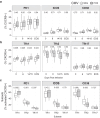
Findings: CMV seropositivity was associated with reduced induction of parasite specific antibodies following malaria infection and vaccination. During infection, reduced antibody induction was associated with modifications to the T -follicular helper (Tfh) cell compartment. CMV seropositivity was associated with a skew towards Tfh1 cell subsets before and after malaria infection, and reduced activation of Tfh2 cells. Protective Tfh2 cell activation was only associated with antibody development in individuals who were CMV seronegative, and a higher proportion of Tfh1 cells was associated with lower antibody development in individuals who were CMV seropositive. During MSP1 vaccination, reduced antibody induction in individuals who were CMV seropositive was associated with CD4 T cell expression of terminal differentiation marker CD57.
Interpretation: These findings suggest that CMV seropositivity may be negatively associated with malaria antibody development. Further studies in larger cohorts, particularly in malaria endemic regions are required to investigate whether CMV infection may modify immunity to malaria gained during infection or vaccination in children.
Funding: Work was funded by National Health and Medical Research Council of Australia, CSL Australia and Snow Medical Foundation. Funders had no role in data generation, writing of manuscript of decision to submit for publication.
Keywords: Antibodies; CD4 T cells; Cytomegalovirus; Malaria.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests RTL is employee from Sumaya GmbH & Co. KG who provide contracts/grants and travel support. All other authors declare no conflicts of interest.
Figures



References
-
- Geneva: World Health Organisation . 2023. World malaria report 2023.
-
- White M.T., Verity R., Griffin J.T., et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis. 2015;15:1450–1458. doi: 10.1016/s1473-3099(15)00239-x. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

