APOA2 increases cholesterol efflux capacity to plasma HDL by displacing the C-terminus of resident APOA1
- PMID: 39490930
- PMCID: PMC11617996
- DOI: 10.1016/j.jlr.2024.100686
APOA2 increases cholesterol efflux capacity to plasma HDL by displacing the C-terminus of resident APOA1
Abstract
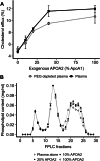
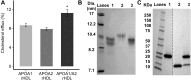
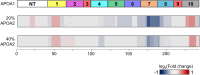
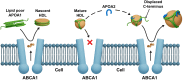
The ability of high-density lipoprotein (HDL) to promote cellular cholesterol efflux is a more robust predictor of cardiovascular disease protection than HDL-cholesterol levels in plasma. Previously, we found that lipidated HDL containing both apolipoprotein A-I (APOA1) and A-II (APOA2) promotes cholesterol efflux via the ATP-binding cassette transporter (ABCA1). In the current study, we directly added purified, lipid-free APOA2 to human plasma and found a dose-dependent increase in whole plasma cholesterol efflux capacity. APOA2 likewise increased the cholesterol efflux capacity of isolated HDL with the maximum effect occurring when equal masses of APOA1 and APOA2 coexisted on the particles. Follow-up experiments with reconstituted HDL corroborated that the presence of both APOA1 and APOA2 were necessary for the increased efflux. Using limited proteolysis and chemical cross-linking mass spectrometry, we found that APOA2 induced a conformational change in the N- and C-terminal helices of APOA1. Using reconstituted HDL with APOA1 deletion mutants, we further showed that APOA2 lost its ability to stimulate ABCA1 efflux to HDL if the C-terminal domain of APOA1 was absent, but retained this ability when the N-terminal domain was absent. Based on these findings, we propose a model in which APOA2 displaces the C-terminal helix of APOA1 from the HDL surface which can then interact with ABCA1-much like it does in lipid-poor APOA1. These findings suggest APOA2 may be a novel therapeutic target given this ability to open a large, high-capacity pool of HDL particles to enhance ABCA1-mediated cholesterol efflux.
Keywords: ABCA1; Apolipoprotein A-I; Apolipoprotein A-II; Apolipoprotein(s); Cholesterol Efflux; High Density Lipoproteins; Lipoprotein metabolism; Lipoproteins; Structure.
Published by Elsevier Inc.
Conflict of interest statement
Conflicts of Interests The authors declare that they have no conflicts of interests with the contents of this article.
Figures


Similar articles
-
Effects of Recombinant Human Lecithin Cholesterol Acyltransferase on Lipoprotein Metabolism in Humans.Arterioscler Thromb Vasc Biol. 2024 Jun;44(6):1407-1418. doi: 10.1161/ATVBAHA.123.320387. Epub 2024 May 2. Arterioscler Thromb Vasc Biol. 2024. PMID: 38695168 Clinical Trial.
-
Functional characterization of missense variants affecting the extracellular domains of ABCA1 using a fluorescence-based assay.J Lipid Res. 2024 Jan;65(1):100482. doi: 10.1016/j.jlr.2023.100482. Epub 2023 Dec 3. J Lipid Res. 2024. PMID: 38052254 Free PMC article.
-
RETRACTED: The effects of Implanon on lipid metabolism in comparison with Norplant.Contraception. 1999 Nov;60(5):281-7. doi: 10.1016/s0010-7824(99)00099-2. Contraception. 1999. Retraction in: Contraception. 2004 Nov;70(5):433. doi: 10.1016/j.contraception.2004.07.004. Retraction in: Contraception. 2025 Aug;148:110947. doi: 10.1016/j.contraception.2025.110947. PMID: 10717780 Retracted. Clinical Trial.
-
High-density lipoprotein cholesterol efflux capacity is inversely associated with cardiovascular risk: a systematic review and meta-analysis.Lipids Health Dis. 2017 Nov 10;16(1):212. doi: 10.1186/s12944-017-0604-5. Lipids Health Dis. 2017. PMID: 29126414 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
Cited by
-
Dietary Intake of a Milk Sphingolipid-Rich MFGM/EV Concentrate Ameliorates Age-Related Metabolic Dysfunction.Nutrients. 2025 Jul 31;17(15):2529. doi: 10.3390/nu17152529. Nutrients. 2025. PMID: 40806113 Free PMC article.
-
Apolipoprotein A (ApoA) in Neurological Disorders: Connections and Insights.Int J Mol Sci. 2025 Aug 16;26(16):7908. doi: 10.3390/ijms26167908. Int J Mol Sci. 2025. PMID: 40869228 Free PMC article. Review.
References
-
- Falk E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006;47(8 Suppl):C7–C12. - PubMed
-
- Favari E., Chroni A., Tietge U.J.F., Zanotti I., Escolà-Gil J.C., Bernini F., von Eckardstein A., Kardassis D., editors. High Density Lipoproteins: From Biological Understanding to Clinical Exploitation. Springer International Publishing; Cham: 2015. pp. 181–206.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

