Notch1 blockade by a novel, selective anti-Notch1 neutralizing antibody improves immunotherapy efficacy in melanoma by promoting an inflamed TME
- PMID: 39491031
- PMCID: PMC11533310
- DOI: 10.1186/s13046-024-03214-5
Notch1 blockade by a novel, selective anti-Notch1 neutralizing antibody improves immunotherapy efficacy in melanoma by promoting an inflamed TME
Abstract
Background: Immune checkpoint inhibitors (ICI) have dramatically improved the life expectancy of patients with metastatic melanoma. However, about half of the patient population still present resistance to these treatments. We have previously shown Notch1 contributes to a non-inflamed TME in melanoma that reduces the response to ICI. Here, we addressed the therapeutic effects of a novel anti-Notch1 neutralizing antibody we produced, alone and in combination with immune checkpoint inhibition in melanoma models.
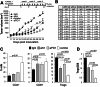
Methods: Anti-Notch1 was designed to interfere with ligand binding. Mice were immunized with a peptide encompassing EGF-like repeats 11-15 of human Notch1, the minimal required region that allows ligand binding and Notch1 activation. Positive clones were expanded and tested for neutralizing capabilities. Anti-Notch1-NIC was used to determine whether anti-Notch1 was able to reduce Notch1 cleavage; while anti-SNAP23 and BCAT2 were used as downstream Notch1 and Notch2 targets, respectively. K457 human melanoma cells and the YUMM2.1 and 1.7 syngeneic mouse melanoma cells were used. Cell death after anti-Notch1 treatment was determined by trypan blue staining and compared to the effects of the gamma-secretase inhibitor DBZ. 10 mg/kg anti-Notch1 was used for in vivo tumor growth of YUMM2.1 and 1.7 cells. Tumors were measured and processed for flow cytometry using antibodies against major immune cell populations.
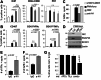
Results: Anti-Notch1 selectively inhibited Notch1 but not Notch2; caused significant melanoma cell death in vitro but did not affect normal melanocytes. In vivo, it delayed tumor growth without evident signs of gastro-intestinal toxicities; and importantly promoted an inflamed TME by increasing the cytotoxic CD8+ T cells while reducing the tolerogenic Tregs and MDSCs, resulting in enhanced efficacy of anti-PD-1.
Conclusions: Anti-Notch1 safely exerts anti-melanoma effects and improves immune checkpoint inhibitor efficacy. Thus, anti-Notch1 could represent a novel addition to the immunotherapy repertoire for melanoma.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, et al. Long-term outcomes with Nivolumab Plus Ipilimumab or Nivolumab alone Versus Ipilimumab in patients with Advanced Melanoma. J Clin Oncol. 2022;40(2):127–37. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

