17α-Ethynylestradiol alters testicular epigenetic profiles and histone-to-protamine exchange in mice
- PMID: 39491035
- PMCID: PMC11533287
- DOI: 10.1186/s12958-024-01307-6
17α-Ethynylestradiol alters testicular epigenetic profiles and histone-to-protamine exchange in mice
Abstract
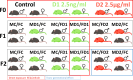
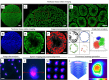
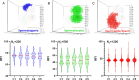
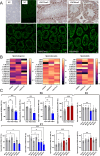
Spermatogenesis starts with the onset of puberty within the seminiferous epithelium of the testes. It is a complex process under intricate control of the endocrine system. Physiological regulations by steroid hormones in general and by estrogens in particular are due to their chemical nature prone to be disrupted by exogenous factors acting as endocrine disruptors (EDs). 17α-Ethynylestradiol (EE2) is an environmental pollutant with a confirmed ED activity and a well-known effect on spermatogenesis and chromatin remodeling in haploid germ cells. The aim of our study was to assess possible effects of two doses (2.5ng/ml; 2.5 μg/ml) of EE2 on both histone-to-protamine exchange and epigenetic profiles during spermatogenesis performing a multi/transgenerational study in mice. Our results demonstrated an impaired histone-to-protamine exchange with a significantly higher histone retention in sperm nuclei of exposed animals, when this process was accompanied by the changes of histone post-translational modifications (PTMs) abundancies with a prominent effect on H3K9Ac and partial changes in protamine 1 promoter methylation status. Furthermore, individual changes in molecular phenotypes were partially transmitted to subsequent generations, when no direct trans-generational effect was observed. Finally, the uncovered specific localization of the histone retention in sperm nuclei and their specific PTMs profile after EE2 exposure may indicate an estrogenic effect on sperm motility and early embryonic development via epigenetic mechanisms.
Keywords: 17α-Ethynylestradiol; DNA methylation; EE2; Endocrine disruptors; Histone-to-protamine exchange; Post-translational modifications; Sperm; Testis; Transgenerational study.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Knobil and Neill’s Physiology of Reproduction, Vols 1 and 2, 3rd Editon. Knobil and Neill’s Physiology of Reproduction, Vols 1 and 2, 3rd Editon, 2006: pp. 1-3238.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

