Intervening to Preserve Function in Ischemic Cardiomyopathy with a Porous Hydrogel and Extracellular Matrix Composite in a Rat Myocardial Infarction Model
- PMID: 39491520
- PMCID: PMC11729544
- DOI: 10.1002/adhm.202402757
Intervening to Preserve Function in Ischemic Cardiomyopathy with a Porous Hydrogel and Extracellular Matrix Composite in a Rat Myocardial Infarction Model
Abstract
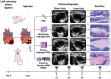
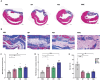
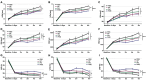
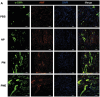
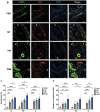
Multiple hydrogels are developed for injection therapy after myocardial infarction, with some incorporating substances promoting tissue regeneration and others emphasizing mechanical effects. In this study, porosity and extracellular matrix-derived digest (ECM) are incorporated, into a mechanically optimized, thermoresponsive, degradable hydrogel (poly(N-isopropylacrylamide-co-N-vinylpyrrolidone-co-MAPLA)) and evaluate whether this biomaterial injectate can abrogate adverse remodeling in rat ischemic cardiomyopathy. After myocardial infarction, rats are divided into four groups: NP (non-porous hydrogel) without either ECM or porosity, PM (porous hydrogel) from the same synthetic copolymer with mannitol beads as porogens, and PME with porosity and ECM digest added to the synthetic copolymer. PBS injection alone is a control group. Intramyocardial injections occurred 3 days after myocardial infarction followed by serial echocardiography and histological assessments 8 weeks after infarction. Echocardiographic function and neovascularization improved in the PME group compared to the other hydrogels and PBS injection. The PME group also demonstrated improved LV geometry and macrophage polarization (toward M2) compared to PBS, whereas differences are not observed in the NP or PM groups versus control. These results demonstrate further functional improvement may be achieved in hydrogel injection therapy for ischemic cardiomyopathy by incorporating porosity and ECM digest, representing combined mechanical and biological effects.
Keywords: extracellular matrix; hydrogel; myocardial infarction; porous structure.
© 2024 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Martin S. S., Aday A. W., Almarzooq Z. I., Anderson C. A. M., Arora P., Avery C. L., Baker‐Smith C. M., Barone Gibbs B., Beaton A. Z., Boehme A. K., Commodore‐Mensah Y., Currie M. E., Elkind M. S. V., Evenson K. R., Generoso G., Heard D. G., Hiremath S., Johansen M. C., Kalani R., Kazi D. S., Ko D., Liu J., Magnani J. W., Michos E. D., Mussolino M. E., Navaneethan S. D., Parikh N. I., Perman S. M., Poudel R., Rezk‐Hanna M., et al., Circulation. 2024, 149, e347. - PMC - PubMed
-
- Pfeffer M. A., Braunwald E., Circulation 1990, 81, 1161. - PubMed
-
- Mann D. L., Bristow M. R., Circulation 2005, 111, 2837. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

