Three-Component 1,2-Dioxygenation of 1,3-Dienes Using Carboxylic Acids and TEMPO
- PMID: 39492589
- PMCID: PMC11574855
- DOI: 10.1021/acs.joc.4c02244
Three-Component 1,2-Dioxygenation of 1,3-Dienes Using Carboxylic Acids and TEMPO
Abstract
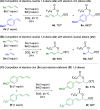
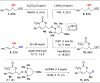
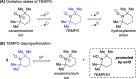
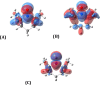
A mild, metal-free 1,2-dioxygenation of 1,3-dienes using TEMPO and carboxylic acids is reported. This method includes examples for a variety of 1,3-dienes as well as aliphatic and aromatic carboxylic acids. This reaction also demonstrates complete site- and regioselectivity in the oxygen addition. Furthermore, extensive derivatization of the differential oxygen groups in the product has been demonstrated, including reduction of the remaining alkene to access alkyl, vicinally dioxygenated scaffolds. Finally, this reaction is shown both experimentally and computationally to occur through carboxylic acid-driven disproportionation of TEMPO.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- McGrath N. A.; Brichacek M.; Njardarson J. T. A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Educ. 2010, 87, 1348–1349. 10.1021/ed1003806. - DOI
-
- Kolb H. C.; VanNieuwenhze M. S.; Sharpless K. B. Catalytic Asymmetric Dihydroxylation. Chem. Rev. 1994, 94, 2483–2547. 10.1021/cr00032a009. - DOI
- Noe M. C.; Letavic M. A.; Snow S. L. Asymmetric Dihydroxylation of Alkenes. Org. React. 2005, 109–625. 10.1002/0471264180.or066.02. - DOI
- Rawling M. J.; Tomkinson N. C. O. Metal-free syn-dioxygenation of alkenes. Org. Biomol. Chem. 2013, 11, 1434–1440. 10.1039/c3ob27387c. - DOI - PubMed
- Bag R.; De P. B.; Pradhan S.; Punniyamurthy T. Recent Advances in Radical Dioxygenation of Olefins. Eur. J. Org. Chem. 2017, 2017, 5424–5438. 10.1002/ejoc.201700512. - DOI
- Wang C. Vicinal anti-Dioxygenation of Alkenes. Asian J. Org. Chem. 2018, 7, 509–521. 10.1002/ajoc.201700621. - DOI
- Bataille C. J. R.; Donohoe T. J. Osmium-free direct syn-dihydroxylation of alkenes. Chem. Soc. Rev. 2011, 40, 114–128. 10.1039/B923880H. - DOI - PubMed
- Colomer I.; Barcelos R. C.; Christensen K. E.; Donohoe T. J. Orthogonally Protected 1,2-Diols from Electron-Rich Alkenes Using Metal-Free Olefin syn-Dihydroxylation. Org. Lett. 2016, 18, 5880–5883. 10.1021/acs.orglett.6b02959. - DOI - PubMed
-
- Clennan E. L. Synthetic and mechanistic aspects of 1,3-diene photooxidation. Tetrahedron 1991, 47, 1343–1382. 10.1016/S0040-4020(01)86413-9. - DOI
- Ghogare A. A.; Greer A. Using Singlet Oxygen to Synthesize Natural Products and Drugs. Chem. Rev. 2016, 116, 9994–10034. 10.1021/acs.chemrev.5b00726. - DOI - PubMed
- de Souza J. M.; Brocksom T. J.; McQuade D. T.; de Oliveira K. T. Continuous Endoperoxidation of Conjugated Dienes and Subsequent Rearrangements Leading to C–H Oxidized Synthons. J. Org. Chem. 2018, 83, 7574–7585. 10.1021/acs.joc.8b01307. - DOI - PubMed
-
- Burks H. E.; Kliman L. T.; Morken J. P. Asymmetric 1,4-Dihydroxylation of 1,3-Dienes by Catalytic Enantioselective Diboration. J. Am. Chem. Soc. 2009, 131, 9134–9135. 10.1021/ja809610h. - DOI - PMC - PubMed
- Ely R. J.; Morken J. P. Ni(0)-Catalyzed 1,4-Selective Diboration of Conjugated Dienes. Org. Lett. 2010, 12, 4348–4351. 10.1021/ol101797f. - DOI - PMC - PubMed
- Schuster C. H.; Li B.; Morken J. P. Modular Monodentate Oxaphospholane Ligands: Utility in Highly Efficient and Enantioselective 1,4-Diboration of 1,3-Dienes. Angew. Chem., Int. Ed. 2011, 50, 7906–7909. 10.1002/anie.201102404. - DOI - PMC - PubMed
- Hong K.; Morken J. P. Catalytic Enantioselective Diboration of Cyclic Dienes. A Modified Ligand with General Utility. J. Org. Chem. 2011, 76, 9102–9108. 10.1021/jo201321k. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

