Optimizing 5-aminosalicylate for moderate ulcerative colitis: expert recommendations from the Asia-Pacific, Middle East, and Africa Inflammatory Bowel Disease Coalition
- PMID: 39492666
- PMCID: PMC11834365
- DOI: 10.5217/ir.2024.00089
Optimizing 5-aminosalicylate for moderate ulcerative colitis: expert recommendations from the Asia-Pacific, Middle East, and Africa Inflammatory Bowel Disease Coalition
Abstract
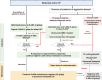
The lack of clear definition and classification for "moderate ulcerative colitis (UC)" creates ambiguity regarding the suitability of step-up versus top-down treatment approaches. In this paper, experts address crucial gaps in assessing and managing moderate UC. The Asia-Pacific, Middle East, and Africa Inflammatory Bowel Disease Coalition comprised 24 experts who convened to share, discuss and vote electronically on management recommendations for moderate UC. Experts emphasized that the goal of treating UC is to attain clinical, biomarker, and endoscopic remission using cost-effective strategies such as 5-aminosalicylates (5-ASAs), well-tolerated therapy that can be optimized to improve outcomes. Experts agreed that 5-ASA therapy could be optimized by maximizing dosage (4 g/day for induction of remission), combining oral and topical administration, extending treatment duration beyond 8 weeks, and enhancing patient adherence through personalized counselling and reduced pill burden. Treatment escalation should ideally be reserved for patients with predictors of aggressive disease or those who do not respond to 5-ASA optimization. Premature treatment escalation to advanced therapies (including biologics and oral small molecules) may have long-term health and financial consequences. This paper provides consensus-based expert recommendations and a treatment algorithm, based on current evidence and practices, to assist decision-making in real-world settings.
Keywords: 5-Aminosalicylates; Inflammatory bowel diseases; Treatment optimization; Ulcerative colitis.
Conflict of interest statement
Akyüz F has received speaker’s fees from AbbVie and Janssen. An YK has received speaking and consulting fees from AbbVie, Bristol Myers Squibb, Celltrion, Chiesi, Dr. Falk, Ferring Pharmaceuticals, Janssen, Pfizer, Sandoz, Shire and Takeda; served on advisory boards member for AbbVie, Bristol Myers Squibb, Chiesi, Janssen, NPS Medicine Wise, Microba; received research and educational funding from AbbVie, Celltrion, Dr Falk, Janssen, Pfizer, Sandoz and Takeda. Begun J has served as an advisory board member, consultant, or speaker for AbbVie, Alimentiv, Bristol Myers Squibb, Celltrion, Eli Lilly, Ferring Pharmaceuticals, Gilead Sciences, Janssen, Chiesi, Anantara, Pfizer, and Takeda. Has received research funding from AbbVie, Pfizer, and Janssen. Aniwan S has received speaker’s fees from Janssen, Ferring Pharmaceuticals, and Takeda, and a fee for attending advisory board/round table discussions with Sandoz and Takeda. Bui HH has received speaker’s fees from Ferring Pharmaceuticals, Janssen and AbbVie. Chan W has received speaker’s fees from AbbVie, Ferring Pharmaceuticals and Janssen. Choi CH has served as an advisory board member for AbbVie Korea, Celltrion, Ferring Pharmaceuticals Korea, Samsung Bioepis, Takeda Korea, and Yuhan, and has received research funding from AbbVie Korea, Celltrion, and Takeda Korea. Chopdat N has no conflict of interest to declare. Connor SJ has received honoraria for advisory board participation, speaker’s fees, educational support and/or research support from: AbbVie, Amgen, Bristol Myers Squibb, Celltrion, Chiesi, Dr. Falk, Eli Lilly, Ferring Pharmaceuticals, GSK, Janssen, MSD, Organon, Pfizer, Sandoz, Takeda, Agency for Clinical Innovation, Medical Research Future Fund, South-Western Sydney Local Health District, Sydney Partnership for Health, Research and Enterprise (SPHERE) and The Leona M and Harry B Helmsley Charitable Trust. Desai D has served as an advisory board member for Ferring Pharmaceuticals. Flanagan E has received speaker’s fees from AbbVie, Ferring Pharmaceuticals, Janssen, Sandoz and Takeda; and received a research grant from Ferring Pharmaceuticals. Kobayashi T has served as an advisory board member, consultant, or speaker for AbbVie, Activaid, Alfresa Pharma, Alimentiv, Bristol Myers Squibb, Celltrion, Covidien, EA Pharma, Eli Lilly, Ferring Pharmaceuticals, Galapagos, Gilead Sciences, Janssen Pharmaceuticals, JIMRO, Kissei Pharmaceutical, Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku, Pfizer, Takeda, and Zeria Pharmaceutical, and has received research funding from AbbVie, Alfresa Pharma, EA Pharma, Gilead Sciences, Kyorin Pharmaceutical, Mochida Pharmaceutical, Nippon Kayaku, Otsuka Holdings, Pfizer, Sekisui Medical, Takeda, and Zeria Pharmaceutical. Lai AYH is an employee of Ferring Pharmaceuticals. Leong RW is an advisory board member of: AbbVie, Aspen, Bristol Myers Squibb, Celgene, Celltrion, Chiesi, Ferring Pharmaceuticals, Glutagen, Hospira, J&J Innovative Medicine, Lilly, Merck Sharpe & Dohme, Novartis, Pfizer, Prometheus Biosciences, Takeda; and is a grant recipient from Celltrion, Shire, Janssen, Takeda, Gastroenterological Society of Australia, Medical Research Future Fund, National Health Medical Research Council, Gutsy Group, Pfizer, Joanna Tiddy Grant University of Sydney and McCusker Charitable Foundation. Leow AHR has received speaker’s fees from AstraZeneca, Takeda, Janssen, Ferring Pharmaceuticals, Abbott and Servier, and fees for attending advisory board/round table discussion with AbbVie and Janssen. Leung WK has received speaker’s fees from AbbVie, Ferring Pharmaceuticals and Janssen; and fees for attending advisory boards of AstraZeneca and Biocodex. Limsrivilai J has received speaker’s fees from Janssen, Ferring Pharmaceuticals, and Takeda, and fees for attending advisory board/round table discussion with Novartis and Takeda. Muzellina VN has received speaker’s fees from Ferring Pharmaceuticals. Ooi CJ has received speaker’s fees from Janssen, Ferring, Dr. Falk, Celltrion and AbbVie, and fees for attending advisory board/round table discussion with AbbVie, Pfizer, Jannsen and Takeda. Peddi K has served as advisory board member to Janssen, Takeda and Ferring Pharmaceuticals. Zhihua R has no conflict of interest to declare. Wei SC has received speaker’s fees from AbbVie, Bristol Myers Squibb, Celltrion, Ferring Pharmaceuticals, Janssen, Pfizer, Takeda and Tanabe, and fees for attending advisory board/round table discussions with AbbVie, Bristol Myers Squibb, Celltrion, Cornerstones, Ferring Pharmaceuticals, Janssen, Pfizer, Sanofi and Takeda. Sollano J has received speaker’s fees from Johnson & Johnson, Celltrion, A Menarini, Ferring Pharmaceuticals and Takeda. Teo MMH is an employee of Ferring Pharmaceuticals. Wu K has served as an advisory board member, consultant, or speaker for AbbVie, Bristol Myers Squibb, Ferring Pharmaceuticals, Gilead Sciences, Janssen Pharmaceuticals, JIMRO, Pfizer, Takeda, IPSEN and BioRey and CMS. Ye BD has served as an advisory board member for AbbVie Korea, Bristol Myers Squibb Pharmaceutical Korea Ltd., Celltrion, Ferring Korea, Janssen Korea, Pfizer Korea, Samsung Bioepis, Takeda, Takeda Korea, and Yuhan; a consultant for Chong Kun Dang Pharm, CJ Red BIO, Curacle, Daewoong Pharm, Dong-A ST, Imscout, IQVIA, Korea Otsuka Pharm, Korea United Pharm, Medtronic Korea, NanoEntek, and ORGANOIDSCIENCES Ltd.; a speaker for AbbVie Korea, Bristol Myers Squibb Pharmaceutical Korea Ltd., Celltrion, Cornerstones Health, Curacle, Eisai Korea, Ferring Korea, Samsung Bioepis, Janssen Korea, Pfizer Korea, and has received research funding from Celltrion, Pfizer Korea, and Takeda Korea.
Ooi CJ and Wei SC are editorial board members of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Figures
References
-
- Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16:2–17. - PubMed
-
- Wang Q, Chen MR, Hao JW, et al. AB1675 Deep phenotyping of immune microenvironment in ulcerative colitis by integrative systems analysis. Ann Rheum Dis. 2023;82:2074–2075.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

