Piezo1 restrains proinflammatory response but is essential in T-cell-mediated immunopathology
- PMID: 39492686
- PMCID: PMC11953072
- DOI: 10.1093/jleuko/qiae242
Piezo1 restrains proinflammatory response but is essential in T-cell-mediated immunopathology
Abstract
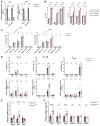
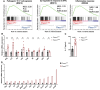
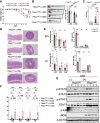
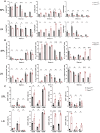
Piezo1 is a mechanosensitive, nonselective Ca2+ channel that is broadly expressed in CD4+ T cells. Using lineage-specific Piezo1 knockout mice (Piezo1cKO), we show that loss of Piezo1 in CD4+ T cells significantly increased IFNγ and IL-17 production in vitro under TH1 and TH17 polarizing conditions, respectively. Despite their intrinsic proinflammatory phenotype, Piezo1cKO T cells are incapable of establishing disease in vivo in 3 separate adoptive transfer T-cell-mediated inflammatory mouse models, including experimental autoimmune encephalomyelitis, inflammatory bowel disease, and graft-vs-host disease. These phenomena coincided with a decreased effector memory (CD44hiCD62Llo) CD4+ T-cell pool derived from donor Piezo1cKO T cells, an observation related to intrinsic T-cell fitness, as a cotransfer inflammatory bowel disease mouse model revealed a deficiency in the CD4+ effector memory population derived only from the naive Piezo1cKO but a not coinfused Piezo1WT CD4+ T-cell source. Taken together, our results support Piezo1 as restraining proinflammatory T-cell differentiation while contributing to the generation and persistence of the effector memory pool during CD4+ T-cell-mediated immunopathology.
Keywords: CD4+ T cell; EAE; T-cell persistence; colitis; functional polarization; gvHD; mechanosensation; piezo1.
© The Author(s) 2024. Published by Oxford University Press on behalf of Society for Leukocyte Biology.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

