Long-term Dynamics of Measles Virus-Specific Neutralizing Antibodies in Children Vaccinated Before 12 Months of Age
- PMID: 39492687
- PMCID: PMC12043057
- DOI: 10.1093/cid/ciae537
Long-term Dynamics of Measles Virus-Specific Neutralizing Antibodies in Children Vaccinated Before 12 Months of Age
Abstract
Background: Measles is a highly contagious disease, presenting a significant risk for unvaccinated infants and adults. Measles vaccination under the age of 12 months provides early protection but has also been associated with blunting of antibody responses to subsequent measles vaccinations and assumed to have lower vaccine effectiveness.
Methods: Our study included children who received an early measles, mumps, and rubella (MMR) vaccination between 6 and 12 months of age (n = 79, given in addition to the regular MMR vaccination schedule at 14 months and 9 years) and a group without additional early vaccination (n = 44). We evaluated measles virus (MeV)-specific neutralizing antibodies before vaccination at 14 months and up to 6 years thereafter using a plaque reduction neutralization test according to the standard set by the World Health Organization.
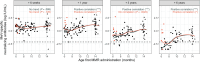
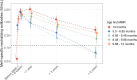
Results: We found a significant association between age of first MMR and MeV-specific neutralizing antibody levels later in life. Although most children who received early vaccination seroconverted after the first dose, children vaccinated before 8.5 months of age exhibited a markedly faster antibody decay and lost their protective neutralizing antibody levels over 6 years.
Conclusions: Routine vaccination of infants under 8.5 months of age may lead to blunted MeV-specific antibody responses to subsequent MMR vaccination. Early MMR vaccination should only be considered during measles outbreaks or in other situations of increased risk of MeV infection. Clinical Trials Registration. EudraCT 2013-003078-28.
Keywords: MMR vaccine; early infant vaccination; immunity; long-term immune protection; measles.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Sindhu TG, Geeta MG, Krishnakumar P, Sabitha S, Ajina KK. Clinical profile of measles in children with special reference to infants. Trop Doct 2019; 49:20–3. - PubMed
-
- Wendorf KA, Winter K, Zipprich J, et al. Subacute sclerosing panencephalitis: the devastating measles complication that might be more common than previously estimated. Clin Infect Dis 2017; 65:226–32. - PubMed
-
- Guerra FM, Crowcroft NS, Friedman L, et al. Waning of measles maternal antibody in infants in measles elimination settings—a systematic literature review. Vaccine 2018; 36:1248–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

