Efficacy and safety of low-dose tetracycline, amoxicillin quadruple therapy in Helicobacter pylori infection: A retrospective single center study
- PMID: 39492823
- PMCID: PMC11525849
- DOI: 10.3748/wjg.v30.i39.4295
Efficacy and safety of low-dose tetracycline, amoxicillin quadruple therapy in Helicobacter pylori infection: A retrospective single center study
Abstract
Background: Helicobacter pylori (H. pylori) eradication rates have declined with the rise of antibiotic-resistant strains in recent years. Although highly effective with a low prevalence of resistance, standard dose tetracycline is associated with frequent adverse events. The efficacy and safety of low-dose tetracycline as part of tetracycline and amoxicillin-containing bismuth quadruple therapy are not well described.
Aim: To compare the efficacy and safety of low-dose compared to standard dose tetracycline with combined amoxicillin-containing bismuth quadruple therapy in patients with H. pylori infection.
Methods: Consecutive patients with H. pylori infection receiving tetracycline, amoxicillin, proton pump inhibitor, and bismuth for 14 days at Sir Run Run Shaw Hospital (1/2022-6/2023) were evaluated. The low-dose tetracycline group received tetracycline 500 mg twice daily (bid) while the standard dose group received 750 mg bid or 500 mg three times daily (tid). Primary endpoints were H. pylori eradication rate and treatment-related adverse events.
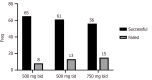
Results: The mean age of the 218 patients was 48.7 ± 14.0 years, 120 (55%) were male, and 118 (54.1%) received treatment as primary therapy. Furthermore, 73 (33%) patients received low-dose tetracycline (500 mg bid) and 145 (67%) received standard dose tetracycline including 500 mg tid in 74 (33%) and 750 mg bid in 71 (33%). On intention-to-treat analysis, H. pylori eradication rates were 89% [95% confidence interval (CI): 82%-96%] in the 500 mg bid group, 82% (95%CI: 74%-91%) in the 500 mg tid group, and 79% (95%CI: 69%-89%) in the 750 mg bid group without a statistically significant difference (P = 0.25). The incidence of adverse events was lower in the low-dose compared to the standard dose group (12.3% vs 31.1% or 23.9%; P = 0.02).
Conclusion: Low-dose tetracycline combined with amoxicillin quadruple therapy for 14 days achieved a high eradication rate and fewer adverse events compared to the standard dose tetracycline regimen in patients with H. pylori infection.
Keywords: Adverse events; Amoxicillin; Bismuth quadruple therapy; Eradication; Helicobacter pylori; Tetracycline.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures
Similar articles
-
Tetracycline Three Times Daily Versus Four Times Daily in Bismuth-Containing Quadruple Therapy as the First-Line Treatment of Helicobacter pylori Infection: A Multicenter, Noninferiority, Randomized Controlled Trial.Helicobacter. 2024 Jul-Aug;29(4):e13121. doi: 10.1111/hel.13121. Helicobacter. 2024. PMID: 39097924 Clinical Trial.
-
Bismuth-Containing Quadruple Therapy for Helicobacter pylori Eradication: A Randomized Clinical Trial of 10 and 14 Days.Dig Dis Sci. 2024 Jul;69(7):2540-2547. doi: 10.1007/s10620-024-08460-3. Epub 2024 May 3. Dig Dis Sci. 2024. PMID: 38700630 Clinical Trial.
-
Amoxicillin or tetracycline in bismuth-containing quadruple therapy as first-line treatment for Helicobacter pylori infection.Gut Microbes. 2020 Sep 2;11(5):1314-1323. doi: 10.1080/19490976.2020.1754118. Epub 2020 May 2. Gut Microbes. 2020. PMID: 32362221 Free PMC article. Clinical Trial.
-
Second-line rescue treatment of Helicobacter pylori infection: Where are we now?World J Gastroenterol. 2018 Oct 28;24(40):4548-4553. doi: 10.3748/wjg.v24.i40.4548. World J Gastroenterol. 2018. PMID: 30386104 Free PMC article. Review.
-
Optimal Duration of Bismuth-Containing Quadruple Therapy for Helicobacter pylori Eradication: A Systematic Review and Meta-Analysis.Helicobacter. 2024 Sep-Oct;29(5):e13144. doi: 10.1111/hel.13144. Helicobacter. 2024. PMID: 39444157
Cited by
-
Challenges and Prospects for Eradication of Helicobacter pylori: Targeting Virulence Factors, Metabolism, and Vaccine Innovation.Pathogens. 2025 Jun 21;14(7):619. doi: 10.3390/pathogens14070619. Pathogens. 2025. PMID: 40732667 Free PMC article. Review.
-
Towards Effective Helicobacter pylori Eradication: Emerging Therapies in the Wake of Antibiotic Resistance.Int J Mol Sci. 2025 Jun 24;26(13):6064. doi: 10.3390/ijms26136064. Int J Mol Sci. 2025. PMID: 40649842 Free PMC article. Review.
References
-
- Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420–429. - PubMed
-
- Liou JM, Malfertheiner P, Lee YC, Sheu BS, Sugano K, Cheng HC, Yeoh KG, Hsu PI, Goh KL, Mahachai V, Gotoda T, Chang WL, Chen MJ, Chiang TH, Chen CC, Wu CY, Leow AH, Wu JY, Wu DC, Hong TC, Lu H, Yamaoka Y, Megraud F, Chan FKL, Sung JJ, Lin JT, Graham DY, Wu MS, El-Omar EM Asian Pacific Alliance on Helicobacter and Microbiota (APAHAM) Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69:2093–2112. - PubMed
-
- Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical