Neuroscience in peripheral cancers: tumors hijacking nerves and neuroimmune crosstalk
- PMID: 39492832
- PMCID: PMC11527832
- DOI: 10.1002/mco2.784
Neuroscience in peripheral cancers: tumors hijacking nerves and neuroimmune crosstalk
Abstract
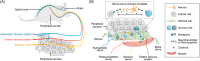
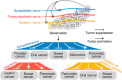
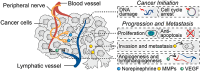
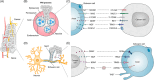
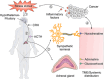
Cancer neuroscience is an emerging field that investigates the intricate relationship between the nervous system and cancer, gaining increasing recognition for its importance. The central nervous system governs the development of the nervous system and directly affects brain tumors, and the peripheral nervous system (PNS) shapes the tumor microenvironment (TME) of peripheral tumors. Both systems are crucial in cancer initiation and progression, with recent studies revealing a more intricate role of the PNS within the TME. Tumors not only invade nerves but also persuade them through remodeling to further promote malignancy, creating a bidirectional interaction between nerves and cancers. Notably, immune cells also contribute to this communication, forming a triangular relationship that influences protumor inflammation and the effectiveness of immunotherapy. This review delves into the intricate mechanisms connecting the PNS and tumors, focusing on how various immune cell types influence nerve‒tumor interactions, emphasizing the clinical relevance of nerve‒tumor and nerve‒immune dynamics. By deepening our understanding of the interplay between nerves, cancer, and immune cells, this review has the potential to reshape tumor biology insights, inspire innovative therapies, and improve clinical outcomes for cancer patients.
Keywords: cancer neuroscience; cancer therapy; neuroimmune crosstalk; peripheral nervous system; tumor microenvironment; tumor‒nerve interactions.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures

References
-
- Magnon C, Hondermarck H. The neural addiction of cancer. Nat Rev Cancer. 2023;23(5):317‐334. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
