Oncolytic viruses alter the biogenesis of tumor extracellular vesicles and influence their immunogenicity
- PMID: 39492948
- PMCID: PMC11530755
- DOI: 10.1016/j.omton.2024.200887
Oncolytic viruses alter the biogenesis of tumor extracellular vesicles and influence their immunogenicity
Abstract
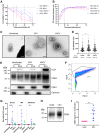
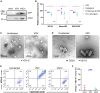
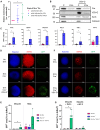
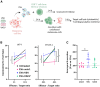
Extracellular vesicles (EVs) are mediators of intercellular communication in the tumor microenvironment. Tumor EVs are commonly associated with metastasis, immunosuppression or drug resistance. Viral infections usually increase EV secretion, but little is known about the effect of oncolytic viruses (OVs) on tumor EVs. Here, we investigated the impact of oncolytic vesicular stomatitis virus (VSV) and vaccinia virus on EVs secreted by human melanoma and thoracic cancer cells. We found that OV infection increases the production of EVs by tumor cells. These EVs contain proteins of viral origin, such as VSV-G, thus creating a continuum of particles sharing markers of both canonical EVs and viruses. As such, the presence of VSV-G on EVs improves the transfer of their protein content to cell types commonly found in the tumor microenvironment. A proteomic analysis also revealed that EVs-OV secreted during VSV infection are enriched in immunity-related proteins. Finally, CD8+ T cells incubated with EVs-OV from infected cells display slightly enhanced cytotoxic functions. Taken together, these data suggest that OVs enhance the communication mediated by tumor EVs, which could participate in the therapeutic efficacy of OVs. These results also provide rationale for engineering OVs to exploit EVs and disseminate therapeutic proteins within the tumor microenvironment.
Keywords: MT: Regular Issue; extracellular vesicles; immunogenicity; immunotherapy; intercellular communication; oncolytic viruses; t cell; vaccinia virus; vesicular stomatitis virus.
© 2024 The Author(s).
Conflict of interest statement
P.E. is an employee of Transgene SA. Transgene SA is a member of the Institut Mérieux Group, a publicly traded French biopharmaceutical company.
Figures


References
-
- Osti D., Del Bene M., Rappa G., Santos M., Matafora V., Richichi C., Faletti S., Beznoussenko G.V., Mironov A., Bachi A., et al. Clinical Significance of Extracellular Vesicles in Plasma from Glioblastoma Patients. Clin. Cancer Res. 2019;25:266–276. doi: 10.1158/1078-0432.CCR-18-1941. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
