Compact Arterial Monitoring Device Use in Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA): A Simple Validation Study in Swine
- PMID: 39493181
- PMCID: PMC11531354
- DOI: 10.7759/cureus.70789
Compact Arterial Monitoring Device Use in Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA): A Simple Validation Study in Swine
Abstract
Introduction Hemorrhage is the leading cause of preventable death in trauma in both the military and civilian settings worldwide. Medical studies from Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) informed change in military prehospital medicine by influencing widespread tourniquet distribution and training on their use to stop life-threatening extremity hemorrhage. In the military setting, there has been a significant reduction in preventable death due to extremity exsanguination since the widespread implementation of tourniquets within the Department of Defense. However, noncompressible hemorrhage remains a significant cause of mortality, especially in the prehospital setting. In select patients, resuscitative endovascular balloon occlusion of the aorta (REBOA) is an adjunct that can be utilized to slow or stop non-compressible hemorrhage until the patient reaches definitive care. However, frontline medical providers face the challenge of reliable, accurate blood pressure measurement in REBOA patients. REBOA, used in conjunction with a small disposable pressure monitor, can bridge the gap in capabilities, creating a more balanced resuscitation and reducing blood product requirements with the added benefit of invasive blood pressure monitoring capability. The authors of this study propose the sustained use and further validation of a small, disposable pressure monitor in REBOA to monitor beat-to-beat variation in both hemodynamically stable and unstable patients and seek to offer a pathway for use in austere environments. Materials and methods Yorkshire swine (n = 4) were selected for partial REBOA (pREBOA) placement and compass transducer measurement in conjunction with a vascular experimental protocol. Appropriate vascular and arterial line access was obtained, hemorrhagic shock was initiated, and REBOA with an in-line Compass™ device (CD) pressure transducer (Centurion Medical Products, Williamston, MI) was used to occlude the aorta. Mean arterial pressures were measured via the CD, recorded, and compared to the control arterial line at hypotensive, normotensive, and hypertensive pressures. Results At hypotensive pressures, 30% of the CD readings fell within 1 mmHg of control arterial line readings, and 52.3% were within 2 mmHg. At normotensive pressures, 46% of the CD readings fell within 1 mmHg of control arterial line readings, and 64.2% were within 2 mmHg. At hypertensive pressures, 60% of the CD readings fell within 1 mmHg of control arterial line readings, and 82% were within 2 mmHg. All CD data points at all pressures were within 8 mmHg of the control arterial line readings. Conclusions In conclusion, the CD is a compact, inexpensive, portable pressure-sensing device that may potentially augment the safety and functionality of the REBOA in trauma patients both at the point of injury and in the hospital. This novel study conducted on four swine subjects demonstrated a remarkable correlation to the traditional equipment intensive arterial line setups, and issues of stasis and non-pulsatility were easily troubleshot. Future studies should investigate CD use in REBOA catheters under different physiological conditions, specifically arrhythmias, and in different environments (prehospital, air medical transport, and austere locations).
Keywords: arterial monitoring; compass transducer; hemodynamic monitoring; partial reboa; swine model.
Copyright © 2024, Lussier et al.
Conflict of interest statement
Human subjects: All authors have confirmed that this study did not involve human participants or tissue. Animal subjects: Institutional Animal Care and Use Committee (IACUC): The research protocol was approved by the IACUC of the Uniformed Services University of the Health Sciences. Ethical approval and Trial Registration Details Per Reviewer Request Below: --------------------------------------------------------------------------------- USUHS / DOD – SPONSORED ANIMAL RESEARCH PROPOSALS MUST USE THIS STANDARDIZED FORMAT Reference DOD Directive 3216.1 & USUHS Instruction 3203 *************************************************************************************Specific information requested in the following animal-use protocol template is a result of requirements of the Animal Welfare Act regulations (AWAR), the Guide for the Care and Use of Laboratory Animals, and other applicable Federal regulations and DOD directives. ************************************************************************************* This document is intended to be an aid in the preparation of a USUHS DOD – sponsored animal use proposal. The instructions and written explanations provided for individual paragraphs (ref. animal-use protocol template in AR 40-33 / USUHSINST 3203, Appendix C) are coded as hidden text. To see the instructions and examples for each section, select the “Show/Hide ¶” button on your tool bar. To print the hidden text, select “Print” on the drop down file menu. Under the “Options” button, select “Hidden text” under the “Include with document” section. Use of a word processor makes completion of this template a “fill-in-the-blanks” exercise. Please provide all response entries in the following font: Arial, Regular, 12, Black. Please do NOT submit this page of instructions with your animal protocol submission. With the exception of title headings, each paragraph and subparagraph in the following template must have a response. Portions of the template that are not applicable to your particular protocol, (i.e., no surgery or no prolonged restraint) should be marked “N/A”. There are no space limitations for the responses. Do not delete any sections. Pertinent standing operating procedures or similar documents that are readily available to your IACUC may be referenced to assist in the description of specific procedures. It is critical that only animal studies or procedures documented in an IACUC – approved protocol be performed at your facility. Additionally, Principal Investigators, or other delegated research personnel, should keep accurate experimental records and be able to provide an audit trail of animal expenditures and use that correlates to their approved protocol. USUHS FORM 3206 ANIMAL STUDY PROPOSAL PROTOCOL COVER SHEET PROTOCOL NUMBER: SUR-19-965 PROTOCOL TITLE: Partial resuscitative endovascular balloon occlusion of the aorta (PREBOA) characterization of targeted distal flow and permissive regional hypoperfusion in a porcine model (Sus scrofa domesticus) of hemorrhagic shock GRANT TITLE (if different from above): NA USUHS PROJECT NUMBER// DAI GRANT NUMBER: Pending FUNDING AGENCY: USU USUHS Form 3206 – Revised February 2018 Previous versions are obsolete EARLIEST ANTICIPATED FUNDING START DATE: Pending PRINCIPAL INVESTIGATOR: Joseph White, MAJ, MC, USA Surgery 301-319-2852 28 Feb 2017 MAJ Joseph White, MD Department Office telephone Date SCIENTIFIC REVIEW: This animal use proposal received an appropriate peer scientific review and is consistent with good scientific research practice. CAPT Eric A. Elster Department Office telephone Date STATISTICAL REVIEW: A person knowledgeable in biostatistics reviewed this proposal to ensure that the number of animals used is appropriate to obtain sufficient data and/or is not excessive, and the statistical design is appropriate for the intent of the study. Statistics USUS 301-295-9468 28 Feb 2017 Cara Olsen, MS, DrPH Department Office telephone Date ATTENDING VETERINARIAN: In accordance with the Animal Welfare Regulations, the Attending Veterinarian was consulted in the planning of procedures and manipulations that may cause more than slight or momentary pain or distress, even if relieved by anesthetics or analgesics. All signatures are required prior to submission to the IACUC Office. LAM 301-295-9492 Anna B. Mullins, DVM, DACLAM Department Office telephone Date BIOSAFETY OFFICER: Only required if using any infectious pathogens. EHS Peter Bouma Department Office telephone Date USUHS Form 3206 – Revised February 2018 Previous versions are obsolete ANIMAL PROTOCOL NUMBER: Pending PRINCIPAL INVESTIGATOR Joseph M. White, MD, FACS MAJ, MC, USA Assistant Program Director, Vascular Surgery Fellowship Assistant Professor of Surgery The Department of Surgery at Uniformed Services University of the Health Sciences and Walter Reed National Military Medical Center Office: 301-319-2852 Email: joseph.m.white70.mil@mail.mil ANIMAL PROTOCOL TITLE: Partial resuscitative endovascular balloon occlusion of the aorta (PREBOA) characterization of targeted distal flow and permissive regional hypoperfusion in a porcine model (Sus scrofa domesticus) of hemorrhagic shock GRANT TITLE (if different from above): NA USUHS PROJECT NUMBER// DAI GRANT NUMBER: Pending CO-INVESTIGATOR(S): Todd E. Rasmussen, MD FACS Colonel USAF MC Shumacker Professor of Surgery Associate Dean for Clinical Research F. Edward Hebert School of Medicine - "America's Medical School" Uniformed Services University of the Health Sciences 4301 Jones Bridge Road Bethesda, Maryland 20814-4712 Office: 301-295-3016 Mobile: 210-508-7062 Email: todd.rasmussen@usuhs.edu Paul W. White, MD, FACS LTC, MC, USA Program Director, Vascular Surgery Fellowship Associate Professor of Surgery, The Department of Surgery at Uniformed Services University of the Health Sciences and Walter Reed National Military Medical Center Office: 301-295-4779 Email: paul.w.white4.mil@mail.mil Thomas A. Davis, PhD Deputy Vice Chair of Research Professor USUHS Form 3206 – Revised February 2018 Previous versions are obsolete USU Walter Reed Surgery Uniformed Services University of the Health Sciences 4301 Jones Bridge Road Bethesda, MD 20814 Office: 301-295-9825 Mobile: 301-204-5212 Email: thomas.davis@usuhs.edu I. NON-TECHNICAL SYNOPSIS: During recent military conflicts, medics and surgeons treat combat trauma patients with critical injuries to the chest, abdomen, and pelvis areas where it can be nearly impossible to control bleeding to save a service member’s life. While the application of tourniquets helps prevent life-threatening blood loss from wounds to extremities (arms and legs), nothing exists for critical injuries to the chest, abdomen, and pelvis in combat and in emergency care environments. A new technology referred to as resuscitative endovascular balloon occlusion of the aorta (REBOA) has been used in trauma patients suffering from rapid blood loss as a result of injuries to their chest, abdomen, and pelvis. This technique involves rapidly placing a flexible catheter into the femoral artery in the groin, maneuvering, and placement in the aorta, and then inflation of an attached balloon at the tip to stop the uncontrolled bleeding. Not surprisingly, total blockage of blood flow to areas to various regions of the body for an extensive time period has resulted in some serious follow-on medical complications. In this proposal, we will test and evaluate the next generation of PREBOA (partial PREBOA) in a well-characterized non-survival swine hemorrhage model. We hypothesize that this new technology will permit longer utilization, enhance organ oxygenation to maintain tissue viability and increase patient survival II. BACKGROUND: II.1. Background: In the current conflicts, many wounded service members have survived catastrophic traumatic injuries. They would have died from these injuries in previous wars, but improvements in battlefield medical care and the use of body armor have increased survival rates. Hemorrhage, particularly non-compressible torso hemorrhage (NCTH), has been identified as a leading cause of preventable death on the modern battlefield.[1,2] Analysis from the recent wars in Iraq and Afghanistan has demonstrated that hemorrhage was the underlying physiologic insult in 90% of potentially survivable battlefield injuries.[3] The stratification of mortality from death on the battlefield from 2001-2011 (Iraq and Afghanistan) demonstrated that 87.3% of all injury mortality occurred in the pre-MTF environment.[3] Of the pre-MTF deaths, 75.7% (n = 3,040) were classified as nonsurvivable, and 24.3% (n = 976) were deemed potentially survivable (PS). The injury/physiologic focus of PS acute mortality was largely associated with hemorrhage (90.9%). The site of lethal hemorrhage was truncal (67.3%), followed by junctional (19.2%) and peripheral-extremity (13.5%) hemorrhage. The management of traumatic hemorrhage (specifically, NCTH) requires innovative strategies and devices to overcome the current trend identified in modern combat. PREBOA seeks to address this critical gap and alter casualty mortality rates as a result. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) has emerged as a promising technique for cases of severe NCTH during trauma. An emerging set of pre-clinical and clinical data on the use of REBOA reports highly successful outcomes following brief periods of occlusion; however, longer periods of occlusion are associated with significantly increased mortality.[4,5] Therapeutic strategies that incorporate regulated partial distal aortic flow and perfusion are a potential technique to mitigate ongoing distal ischemia and subsequent reperfusion injury while potentially avoiding the deleterious USUHS Form 3206 – Revised February 2018 Previous versions are obsolete complications of distal clot disruption with ongoing hemorrhage and supraphysiologic central and cerebral pressures.[6] In order to allow distal aortic flow and perfusion at a controlled and regulated level, REBOA distal flow characteristics require additional pre-clinical testing and evaluation. The PREBOA-PRO Catheter utilizes the same basic technological characteristics as the existing ER-REBOA Catheter – complete large vessel occlusion and blood pressure monitoring – with minor modifications to the balloon design to help facilitate a smoother transition between full occlusion and no occlusion. The PREBOA-PRO Catheter employs a composite balloon design that arranges a non-compliant partner or “spine” balloon in parallel with a compliant occlusion balloon. When the balloon system is fully inflated, complete occlusion is achieved. During balloon deflation, bypass channels initially open around the partner balloon, allowing a limited amount of blood to flow past the balloon, while maintaining balloon wall apposition Figure 1). This permits modulation of blood pressure gradient across the balloon during inflation/deflation cycles. Partial REBOA is a a technique, which shows great promise to reduce morbidities associated with survival from REBOA and extend the amount of time in which REBOA can be performed. REBOA is a resuscitative adjunct that augments central and cerebral perfusion while mitigating lethal hemorrhage secondary to NCTH. Next generation Prytime Medical Devices Inc. partial REBOA (PREBOA-PRO) catheter is currently not FDA-approved for use. Standard REBOA catheter systems are currently in use in military Role I, II, and III facilities and Medical Treatment Facilities. Forward elements such as GHOST-T (Golden Hour Offset Surgical Treatment-Teams) and SOST (Special Operations Surgical Teams) have incorporated and implemented REBOA technology on the forward edge of the battlefield. Civilian level 1 trauma centers currently use REBOA catheter systems. Clinical use typically employs “all-or-nothing” REBOA, meaning that the balloon is fully inflated or completely deflated. PREBOA allows for distal aortic flow, potentially expanding the resuscitation envelope. This would represent lengthening the “golden hour” and augmenting austere or prolonged field care. Permissive Regional Hypoperfusion (PRH) following complete aortic occlusion incorporates regulated partial distal aortic flow and perfusion with subsequent mitigation of distal ischemia and reperfusion injury. This concept potentially avoids the deleterious complications of distal clot disruption with ongoing hemorrhage and supraphysiologic central and cerebral pressures. Building upon the knowledge acquired during previous REBOA testing: REBOA Distal Flow Dynamics and Targeted Distal Flow Characterization (which characterizes the flow-volume relationship and TDF fidelity), this project attempts to identify the critical flow threshold at which Permissive Regional Hypoperfusion (PRH) allows for optimal metabolic and physiologic results. The overarching goal is to decrease morbidity and mortality in the out-of-hospital (prehospital, en-route and far forward medicine) environment scenarios using a partial occlusive endovascular device (pREBOA) to lessen hypotension and ischemia-reperfusion injury while augmenting pulmonary and cerebral perfusion. In this study, we will compare several state-of the-art intravascular occlusion devices in a swine model of lethal non-compressible torso hemorrhage (NCTH). Figure 1: The pREBOA-PROTM Catheter USUHS Form 3206 – Revised February 2018 Previous versions are obsolete II.2. Literature Search for Duplication: II.2.1. Literature Source(s) Searched: PubMed, DTIC, and NIH RePORT were searched to avoid unnecessary duplication of research. II.2.2. Date of Search: 8 January 2018 II.2.3. Period of Search: 1990 to present II.2.4. Key Words and Search Strategy: Separate search terms: PREBOA (partial resuscitative endovascular balloon occlusion of the aorta) in a large animal model (9) REBOA (resuscitative endovascular balloon occlusion of the aorta) in a large animal model (14) Combined search terms: PREBOA (partial resuscitative endovascular balloon occlusion of the aorta) in a large animal model AND Sus scrofa domesticus (7) REBOA (resuscitative endovascular balloon occlusion of the aorta) in a large animal model AND Sus scrofa domesticus (9) PREBOA (partial resuscitative endovascular balloon occlusion of the aorta) in a large animal model AND Hemorrhagic shock (7) REBOA (resuscitative endovascular balloon occlusion of the aorta) in a large animal model AND Hemorrhagic shock (9) (Please see appendix A) II.2.5. Results of Search: Summary: No studies were identified in the literature that would appear to duplicate the proposed large-animal research project. The PREBOA-PRO prototype to be evaluated is novel, and no duplicative in vivo studies were identified. Please see Appendix A for Search Results. III. OBJECTIVE\HYPOTHESIS: The objectives of this study are two-fold (1) to characterize distal aortic flow following PREBOA using the ER-REBOA, and PREBOA-PRO catheters in an established, translational, swine model of lethal NCTH and (2) to identify the critical threshold at which Permissive Regional Hypoperfusion (PRH) allows for reduced perfusion pressure promoting clot stabilization (avoidance of hemorrhage), mitigates tissue ischemia and reperfusion injury, and dampens supraphysiologic central and cerebral pressures. We hypothesis the PREBOA-PRO catheters will demonstrate superior Targeted Distal Flow (TDF) fidelity compared to the ER-REBOA and that a TDF of 150mL/min will demonstrate. USUHS Form 3206 – Revised February 2018 Previous versions are obsolete clot stabilization (avoid free intraperitoneal hemorrhage), mitigate distal ischemia and reperfusion injury and result in a reduction of supraphysiologic central and cerebral pressures thereby demonstrating superior Permissive Regional Hypoperfusion (PRH). IV. MILITARY RELEVANCE: Eastridge and colleagues reported that hemorrhage is the leading cause of preventable death on the modern battlefield. Between October 2001 and June 2011, 4,596 battlefield fatalities were reviewed and analyzed during the wars in Iraq and Afghanistan. The stratification of mortality demonstrated that 87.3% of all injury mortality occurred in the pre-MTF (Medical Treatment Facility) environment. Of the pre-MTF deaths, 75.7% (n = 3,040) were classified as nonsurvivable, and 24.3% (n = 976) were deemed potentially survivable (PS). The injury/physiologic focus of PS acute mortality was largely associated with hemorrhage (90.9%). The site of lethal hemorrhage was truncal (67.3%), followed by junctional (19.2%) and peripheral-extremity (13.5%) hemorrhage.[3] The management of hemorrhage is critical to improving combat causality care (CCC) in the military. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) has emerged as a promising technique for cases of severe NCTH during trauma. Prompt control of bleeding and resuscitation in the field can reduce mortality by as much as 20%. Importantly, hemorrhage is a major mechanism of death in potentially survivable combat injuries, underscoring the necessity for initiatives to mitigate bleeding and to develop new solutions to provide for prolonged Damage Control Resuscitation (pDCR), particularly in the prehospital-en route and prolonged field care (PFC) austere environment. Treatments initiated within the “Golden Hour”- 60 minutes post-injury have resulted in increased survival. REBOA devices provide a short-term survival advantage by preventing exsanguination and augmenting perfusion of the heart, lungs, and brain. However, experimental observation suggests that complete aortic occlusion using REBOA can lead to severe multifactorial complications, including myocardial dysfunction, respiratory distress, traumatic brain injury, and distal organ/extremity ischemia-reperfusion injury, resulting in multi-organ dysfunction and death. Therapeutic strategies that incorporate regulated partial distal aortic flow and perfusion are a potential technique to mitigate ongoing distal ischemia and subsequent reperfusion injury while potentially avoiding the deleterious complications of distal clot disruption with ongoing hemorrhage and supraphysiologic central and cerebral pressures. In order to allow distal aortic flow and perfusion at a controlled and regulated level, REBOA distal flow characteristics require additional investigation. V. MATERIALS AND METHODS: V.1. Experimental Design and General Procedures: General Overview/Concept Specific Aims Model Development Animals: Two animals from each experimental group will be considered a pilot study for each named study. These animals will be used to teach and perfect various aspects procedures associated with two proposed animal models (Section V.4.3.2. Procedure) to include but not limited to the surgical preparation, sedation, intubation, anesthesia monitoring, cut downs, placement of arterial and venous lines, injury induction, REBOA insertion and placement, radiographic assessments, as well clinical and laboratory monitoring. USUHS Form 3206 – Revised February 2018 Previous versions are obsolete Experiment 1: In a controlled prefixed bleed hemorrhagic shock model, evaluate and characterize distal aortic flow rate capabilities with the ER-REBOA, PREBOA-PRO (N=2 pilot animals + 8 animals per treatment arm; total 18 animals). Experiment 2: Following complete aortic occlusion and PRH in a swine traumatic liver amputation uncontrolled bleed hemorrhagic shock model (approximately 30% of the liver followed by 1.5 minutes of free hemorrhage), evaluate and characterize associated hemodynamics and metabolic/physiologic consequences of TDF at 50 mL/min (Grp 1), 150 mL/min (Grp 2), 300 mL/min (Grp 3), 500 mL/min (Grp 4) and 1000 mL/min (Grp 5). N=2 pilot animals + 8 animals per treatment arm; total 42 swine. (*Please see section V.4.3.2. Procedure for additional operative details regarding the specific sequence of events/conduct of the operation) V.1.1. Experiment 1: Target Distal Perfusion (18 animals) (USDA pain category D) Experimental Design 1: Uncontrolled bleed hemorrhagic shock (HS) will be simulated by withdrawing 25% of estimated blood volume through an arterial sheath into citrated blood collection bags over a 30-minute period to simulate the prehospital environment. Complete aortic occlusion will be initiated by inflating the aortic occlusion balloon catheter until there is loss of a distal arterial waveform and occlusion will be sustained for 20 minutes. After 15 minutes of occlusion, shed blood volume will be returned through the central venous line via rapid infusion (Belmont Instruments, Billerica, MD) at the rate of 150 cc/min. Following 20 minutes of Zone I aortic occlusion, incremental balloon deflation at a rate of 0.5 cc every 30 seconds will occur until the balloon is completely deflated. Physiologic parameters and aortic flow measurements will be collected. Onset of aortic flow during balloon deflation will be defined as the time point when flow reaches 50 mL/min (approximately 2% of baseline full flow) to account for the fidelity of the aortic flow probe at very low flow rates. The estimated time for incremental balloon deflation is 15-20 minutes (average balloon total volume: 5.25cc-7.25cc). Upon completion of the incremental balloon deflation, the subsequent phase of testing will determine balloon fidelity and the ability to provide a targeted distal flow. Using data extracted from the initial phase of the experiment, a balloon volume that corresponds with a targeted distal flow of 150ml/min will be selected. The aortic occlusion balloon catheter will be inflated and targeted distal flow recorded over a 90 minute period. At the end of the experiment, the animals will be humanely euthanized according to V.4.6. V.1.2. Experiment 2: Permissive Regional Hypoperfusion (42 animals) (USDA pain category D) Experimental Design 2: HS shock will be initiated by traumatic amputation of the liver (approximately 30% total liver volume) followed by 1.5 minutes of free hemorrhage. The liver will be marked along the planned transection plane, 2 cm to the left of Cantlie's line, to provide amputation of approximately 80% of the left lateral lobe of the liver and 40% of the left medial lobe of the liver (approximately 30% of total liver volume) similar to previous descriptions. At Time 0, the liver will be sharply transected, and the abdomen rapidly closed with cable ties. Complete occlusion of the aorta will be achieved with REBOA 1.5 minutes following the initiation of injury. Complete aortic occlusion will be initiated by inflating the PREBOA-Pro until there is loss of a distal arterial waveform and occlusion is sustained for 20 minutes. Following 20 minutes of Zone I aortic occlusion, PRH with partial aortic occlusion will occur. The pREBOA will be deflated to achieve a distal aortic flow of 50mL/min (Group 1), 150mL/min (Group 2), USUHS Form 3206 – Revised February 2018 Previous versions are obsolete 300mL/min (Group 3), 500mL/min (Group 4) and 1000 mL/min (Group 5). The variable deflation of occlusion balloon will correlate to the goal TDF specific to Groups 1-5. PRH phase will continue for 70 minutes. Next, study animals will undergo a damage control surgery with definitive hemorrhage control and resuscitation with whole blood. Blood volume will be returned through the central venous line via rapid infusion (Belmont Instruments, Billerica, MD) at the rate of 150 cc/min. The REBOA catheter will be removed and the study animals will complete a 240- minute critical care phase (continued hemodynamic monitoring and resuscitation). At the end of the experiment, the animals will be humanely euthanized according to V.4.6. 2 Animals are for pilot. V.2. Data Analysis: Experiment 1: Two endovascular balloon occlusion devices (ER-REBOA and PREBOA-PRO) will be tested and evaluated. The association between flow and volume will be assessed for each experimental animal using linear regression with flow as the dependent variable and volume as the independent variable. For overall comparisons, the separate regressions will be combined across animals and compared between groups using a multilevel regression model with subject-specific random intercepts and slopes, and fixed effects of group, volume, and the interaction of group and volume. A primary hypothesis is that the slope (the increase in flow corresponding to a specific decrease in volume) will be greater in the ER-REBOA group than in the PREBOA-PRO (next generation REBOA catheter) group. With a sample size of 8 animals per group and an average of 15 measurements per animal, if the within-animal correlation is 0.5, the study will have 80% power to detect a significant difference if, across the range of volumes, the increase in flow is lower in the PREBOA-PRO group than the ER-REBOA group by 0.74 standard deviations. If the standard deviation of the distal flow rate is close to 300 mL/minute, as has been observed in similar studies, this corresponds to a result in which the difference in flow rates from the lowest to the highest volume is reduced in the PREBOA-PRO group by 222 mL/minute compared to the ER-REBOA group. For testing whether the variability in flow level over time differs between groups, the within- subject standard deviation will be calculated for each animal and these standard deviations will be compared between groups using a Mann-Whitney U test. Although we have no prior data on the within-subject standard deviation, the power of the Mann-Whitney U test is expected to be similar to that of a corresponding Student's t test. With 8 animals per group, the study will have 80% power to detect a difference of 1.5 standard deviations. Actual power may be slightly lower but is still expected to be adequate. Experiment 2: Five distal flow rates will be evaluated in order to determine optimal regional permissive hypotension following partial REBOA in a model of lethal hemorrhagic shock. Mortality will be compared across groups using Fisher's exact test. When comparing the flow rate with the highest vs. lowest mortality rate, if mortality is 90% vs. 10% and the sample size is 8 per group, Fisher's exact test with a 5% two-sided significance level will have 80% power to detect a difference. If a Bonferroni adjustment is used to account for pairwise comparisons among 5 groups (flow rates) the power will be 80% to detect a significant difference if the true rates are 95% and 5%. Therefore, the sample size is sufficient to detect the expected large differences in mortality. Histologic, physiologic and metabolic parameters will be compared across groups using one- way ANOVA followed by Tukey's pairwise comparisons. A sample size of 8 per group will have 80% power to detect differences of 1.9 standard deviations between groups based on ANOVA contrasts with 5% two-sided significance level after adjustment for multiple comparisons. USUHS Form 3206 – Revised February 2018 Previous versions are obsolete V.3. Laboratory Animals Required and Justification: V.3.1. Non-animal Alternatives Considered: No feasible non-animal alternatives are available to study PREBOA physiology with metabolic and physiologic endpoints. V.3.2. Animal Model and Species Justification: Swine species allows for significant advantage due to the animals arterial capacity (i.e. arterial size and structural similarity to human arteries) to allow for and facilitate most endovascular devices and procedures. Specifically, the porcine common femoral artery can accommodate 7 French arterial sheaths (the required arterial sheath size for the PREBOA-PRO and ER- REBOA-PRO catheter). Additionally, histologic similarities between swine and human aneurysmal models are favorable. V.3.3. Laboratory Animals ALTERNATIVES CONSIDERATIONS: Does the protocol have any provisions that would qualify it to be identified as one that Refines, Reduces, or Replaces (3R's) the use of animals in relation to other protocols or procedures performed in the past? Y/N (circle): YES SECTION V.3.5. Exceptions to the Guide for the Care and Use of Laboratory Animals (Please check all applicable): Use of Paralytics (V.4.1.2.3.) Prolonged Restraint (V.4.2.) Multiple Major Survival Surgery (V.4.3.6.) Use of Non-pharmaceutical grade chemicals (V.4.4.1.) Use of Complete Freund’s Adjuvant (V.4.4.3.) Death as an endpoint (V.4.5.) Food/Water Restriction (V.5.1.2. Single Housing of Social Species (V.5.1.3) Restriction of Environmental Enrichment (V.5.3.2.) [x] Drug Use/Controlled Substances (Appendix B) Use of any Infectious Pathogen (Cover Page and VII.) IDENTIFICATION OF SPECIES AND STRAIN: In accounting for animal numbers, please ensure that the strain of animal as well as the species is identified. If more than one strain of any species will be used, please list each proposed strain in a separate column. If more than two species/strains are to be used, duplicate Sections V.3.3.1 – V.3.4 , and Section V.4.1.1.1, on subsequent pages to cover all requested strains. Species #1 V.3.3.1. Genus & Species: Sus scrofa domesticus V.3.3.2. Strain/Stock: Yorkshire swine USUHS Form 3206 – Revised February 2018 Previous versions are obsolete V.3.3.3. Source/Vender: ABI V.3.3.4. Age: Adult V.3.3.5. Weight: 70-100 kg V.3.3.6. Sex: Female V.3.3.7. Special Consideration: None V.3.4. Number of Animals Required (by Species): Sus scrofa domesticus: 60 V.3.5. Refinement, Reduction, Replacement (3 Rs): V.3.5.1. Refinement: General anesthesia will be used for all surgical procedures. All surgical procedures will be performed by skilled surgeons. All animals will receive buprenorphine pre-operatively to ensure pain is well managed during surgery. V.3.5.2. Reduction: No adaptive model would be possible to decrease the number of animals required. Please see power analysis (Section V.2 Data Analysis) as to how the power analysis was structured and established. Computer models not applicable. V.3.5.3. Replacement: None: Non-animal models cannot replace the animal model for this study. The intact physiology of the whole living animal is required to attain the goals outlined in this study. V.4. Technical Methods: V.4.1. Pain / Distress Assessment: V.4.1.1. APHIS Form 7023 Information: V.4.1.1.1. Number of Animals: V.4.1.1.1. 1. Column C: V.4.1.1.1. 2. Column D: 18 Experiment #1, 42 Experiment #2, Total 60 V.4.1.2. Pain Relief / Prevention: V.4.1.2.1. Anesthesia/Analgesia/Tranquilization: Anesthesia is induced using ketamine (33mg/kg) intramuscularly and maintained with 1% to 4% inhaled isoflurane. General anesthesia will be used for all surgical procedures. Pre- operative pain medication is buprenorphine at 0.01-0.05 mg/kg given SQ at rate of every 4-12 USUHS Form 3206 – Revised February 2018 Previous versions are obsolete hrs. Additional pain medication options include carprofen (2 mg kg/SC) or meloxicam (0.4mg/kg SC). V.4.1.2.2. Pre- and Post-procedural (not surgery) Provisions: NA V.4.1.2.3. Paralytics: None V.4.1.3. Literature Search for Alternatives to Painful or Distressful Procedures: No reasonable alternatives are available to study V.4.1.3.1. Sources Searched: The USDA site (http://www.nal.usda.gov/awic/databases/databse.htm) was searched for possible alternatives. V.4.1.3.2. Date of Search: December 2017 V.4.1.3.3. Period of Search: 1990 – Present V.4.1.3.4. Key Words of Search: Combined search terms: Swine AND resuscitative endovascular balloon occlusion of the aorta AND Alternatives to Painful or Distressful Procedures (0) V.4.1.3.5. Results of Search: Summary: We were unable to find alternatives to the painful/distressful procedures in this protocol because the intact physiology of the whole living animal is required to attain the goals outlined in this study. Please see Appendix A for Search Results V.4.1.4. Unalleviated Painful or Distressful Procedure Justification: NA V.4.2. Prolonged Restraint: None V.4.3. Surgery: *Please see Sections V.1 and V.4.3.2. V.4.3.1. Pre-surgical Provisions: Pigs will receive no food after 1500 hrs on the day before the procedure, with fasting from at least 12 hrs to no more than 24 hrs prior to surgery. V.4.3.2. Procedure: USUHS Form 3206 – Revised February 2018 Previous versions are obsolete A. General Considerations for Animal Preparation Yorkshire-cross swine (Sus scrofa), will be premedicated with Ketamine intramuscularly. Following isoflurane induction and endotracheal intubation, maintenance anesthesia will consist of 1-4% isoflurane in 100% oxygen. To offset the vasodilatory effects of general anesthesia, an intravenous infusion of norepinephrine (0.01 μg/kg per hour) will be instituted upon venous access and titrated to achieve a target MAP between 65 and 75 mm Hg. The infusion rate will remain constant for the remainder of the experiment. Animals will be mechanically ventilated with tidal volumes of 7 to 10 mL/kg and a respiratory rate of 10 to 15 breaths per minute sufficient to maintain end-tidal CO2 at 40 ± 5 mm Hg. Isotonic sodium chloride solution will be administered at a rate of 5 mL/kg per hour to overcome insensible losses. An underbody warmer set at 39°C will be used to maintain body temperature. Venous access will be established through a central line in the right internal jugular vein (percutaneous access). Proximal and distal arterial access will be obtained through a 7 Fr 13-cm introducer sheath (Boston Scientific, Marlborough, MA) placed in the right femoral artery and left brachial (axillary) artery via surgical cut-downs. The animals will undergo midline laparotomy and splenectomy to minimize hemodynamic variation from autotransfusion. Foley catheter will be placed through open surgical cystotomy. The distal aorta will then exposed by longitudinally dividing the retroperitoneal tissues to facilitate placement of a periaortic flow probe (Transonic Systems Inc, Ithaca, NY). The proximal flow probe will be placed at the common carotid artery. The abdomen was closed with cable ties. B. Vascular Exposure and Monitoring Lines A 5 Fr microcatheter (Cook Medical, Inc., Bloomington, IN) will be placed into the brachial artery for hemodynamic monitoring and to facilitate required laboratory draws. This catheter will be inserted along the medial aspect of the front limb. Using the palpable pulse as a landmark, the brachial artery will be dissected out sharply through a longitudinal incision. Care will be taken to avoid the small lateral branches, and silk ties will be used to isolate the vessel. The micocatheter will be inserted following needle and guidewire placement in a modified Seldinger fashion. The catheter will be secured in place using the distal silk tie. Additional anchoring sutures will then be placed to avoid catheter dislodgement. C. Carotid Artery Exposure for Proximal TRANS-SONIC Flow Probe (TSFP) placement Carotid artery exposure will commence with an incision over the anterior border of the sternocleidomastoid. The dissection will be carried through the skin and subcutaneous tissue to the level of the platysma. The platysma will be divided using electrocautery. The sternocleidomastoid will be identified and reflected laterally. The jugular vein and its nodal packet will be identified. The jugular vein will be reflected laterally along with the nodal packet to expose the carotid sheath. Venous tributaries will be divided between heavy silk sutures if required. The common carotid will be identified and circumferentially isolated from surrounding tissues. A Rummel tourniquet will be positioned at the proximal common carotid artery and large vessel-loop placed at the distal common carotid artery. Next, the proximal TSFP will be secured into position. D. Laparotomy with Splenectomy and Cystotomy The operation will commence with a standard midline laparotomy incision. The dissection will be carried through the dermis and subcutaneous tissue to the level of the fascia. The fascia will be entered in the midline. The incision will be extended to the xiphoid cranially and to the pubic symphysis caudally. An Omni retractor versus Balfour retractor will be placed to retract the abdominal wall. The small bowel will be collected into a bowel bag and placed toward the animals right-side. The spleen vasculature will be isolated (does not required mid-line mobilization). The major and minor splenic pedicles will be suture-ligated and splenectomy USUHS Form 3206 – Revised February 2018 Previous versions are obsolete completed. Next, the bladder will be visualized. A small cystotomy will be created sharply and standard Foley catheter placed. The Foley will be sutured into position. E. Infrarenal Abdominal Aortic Exposure for Distal TRANS-SONIC Flow Probe (TSFP) placement A midline laparotomy incision will be completed in standard fashion. The animal’s visceral contents will be mobilized in a similar fashion to human aortic surgery in order to facilitate wide exposure of the infrarenal abdominal aorta. This typically requires cranial mobilization of the transverse colon and lateral retraction of the small bowel to the animal’s right side. The large swine spleen will be removed as previously described. The small bowel will be gathered into a bowel bag and placed within the right lateral aspect of the animal’s peritoneal cavity. The retroperitoneal tissues overlying the aorta will be divided exposing the underlying aorta. The renal arteries will be identified and will serve as the cranial extent of the surgical field. Proximal and distal control will be obtained through circumferential dissection and isolation. The distal TSFP will be sized to the animals’ aorta at the trifurcation. Following circumferential dissection and isolation, the flow probe will be placed. F. Alternative Retroperitoneal Exposure of Right External Iliac Vessels (if required) The iliac vessels will be exposed using a standard retroperitoneal approach through a right transverse-oblique incision. Dissection will proceed in standard fashion through the layers of the abdominal wall until the peritoneum is identified. The peritoneal sac is mobilized away from the abdominal sidewall using gentle blunt dissection. Once the iliac artery and vein are exposed, the cephalad lymphatic tissue overlying the artery is swept medially. Proximal and distal exposure is obtained and vessel loops are placed around the external iliac artery. G. Percutaneous Common Femoral Artery Access Ultrasound-guided percutaneous access will be achieved at the common femoral artery (RIGHT versus LEFT) utilizing the 5-French micro-puncture system in a retrograde fashion following radiographic identification of the femoral head. Next, the micro-system sheath will be exchanged for a standard 7 French sheath. The 7 F sheath will be secure into place with 3-0 nylon suture. Non-survival Exploratory Laparotomy The non-survival exploratory laparotomy (NSEL) will be completed at the index operation. The NSEL will be completed through the previous midline laparotomy incision. Final laboratory evaluation (please see Outcome Measures for list of variables) and physiologic/metabolic markers will be completed. The animal will be euthanized. Next, tissue samples from the renal parenchyma, myocardium, liver, small bowel, hind limb muscle and brain will be collected. Outcome Measures Vital signs – including monitoring of central hemodynamics, pulmonary artery pressure, cardiac output and cardiac index – and other physiologic monitoring such as core body temperature, mean arterial pressure (MAP), ventilation rate, heart-rate, LEFT heart function. CBC, chemistries, arterial blood gases, measures of coagulation (ROTEM). Total blood loss will be monitored in hemorrhaged animals and resuscitation requirements during recovery phase of the experiments will also be measured. Mortality of the model and within the various study groups will be assessed and all animals will undergo necropsy (following death or euthanasia) with tissue samples from renal parenchyma, myocardium, liver, small bowel, hind limb muscle and brain preserved in both RNA. Later tissue preservation medium (gene mRNA transcript expression analysis using RT-PCR) and in 10% buffered formalin solution for embedded in USUHS Form 3206 – Revised February 2018 Previous versions are obsolete paraffin, sectioned and stained with hematoxylin and eosin (H&E) for histopathological assessment by a pathologist blinded to the intervention. V.4.3.3. Post-surgical Provisions: NA V.4.3.4. Location: LAM Surgery Room as assigned V.4.3.5. Surgeons: Surgeon (Attending Vascular Surgeons) Todd Rasmussen, MD Joseph White, MD V.4.3.6. Multiple Major Survival Operative Procedures: V.4.3.6.1. Procedures: No additional procedures V.4.3.6.2. Scientific Justification: NA V.4.4. Animal Manipulations: V.4.4.1. Injections: All injected agents will be Pharmaceutical Grade products.The diluent used will be Normal Saline if needed. Drug Dose Conc Route Approx Volume Frequenc y Site Needle size Ketamine 33 mg/kg 100 mg/mL IM or SC 6-9 mL Once Thigh or neck 18-23ga 0.5-1 in Atropine 0.05 mg/kg 0.54 mg/mL IM or SC 0.5-2 mL Once Thigh or neck 18-23ga 0.5-1 in Euthanasia solution 100 mg/kg Per label IV or IC 4-6 mL Once Catheter or IC 20-23ga 0.5-1 in (IC 20- 23ga 2-2.5”) Buprenorphine 0.01- 0.05mg/kg Per label IM or SC 1ml q4-12hrs Thigh or neck 18-23ga 0.5-1 in Carprofen 2-4mg/kg 50mg/ml SC 3-4ml As needed q12hrs Thigh or neck 18-23ga 0.5-1 in Meloxicam 0.1-0.4mg/kg 5mg/ml SC 1.5-2ml As needed q 24hrs Thigh or neck 18-23ga 0.5-1 in Norepinephrine 0.01ug/kg/hr Per label IV Calculated based on length of surgical procedures CRI Catheter 20-23ga 0.5-1 in V.4.4.2. Biosamples: CBC Clinical chemistries Arterial blood gases Measures of coagulation (ROTEM) Tissue Collections: following death or euthanasia, all animals will undergo necropsy with tissue samples collected from the renal parenchyma, myocardium, liver, small bowel, hind limb muscle and brain preserved in both RNALater and formalin. Tissues preserved in RNALater tissue preservation medium will be assessed for gene mRNA transcript expression analysis using RT-PCR and tissues preserved in 10% buffered formalin solution will be embedded in USUHS Form 3206 – Revised February 2018 Previous versions are obsolete paraffin, sectioned and stained with hematoxylin and eosin (H&E) for histopathological assessment by a pathologist blinded to the intervention. V.4.4.3. Adjuvants: None V.4.4.4. Monoclonal Antibody (MAbs) Production: None V.4.4.5. Animal Identification: Cage cards, animal numbers/tags V.4.4.6. Behavioral Studies: NA V.4.4.7. Other Procedures: All procedures described in section V.4.3.2. V.4.4.8. Tissue Sharing: Any and all remaining available tissues or samples from this protocol may be used by other investigators after the appropriate necropsy has been completed. V.4.5. Study Endpoint: Experiment 1 (Non-survival experiment): Completion of surgical intervention with identification of TDF. Animals will be euthanized in accordance with (IAW) V.4.6. Estimated time from induction of anesthesia to euthanasia is 150 minutes. Experiment 2 (Non-survival experiment): Completion of SICU intervention following surgery with PRH. Animals will be euthanized IAW V.4.6. Estimated time from induction of anesthesia to euthanasia is 360 minutes. V.4.6. Euthanasia: Euthanasia methods will be IAW the most current AVMA Guidelines for the Euthanasia of Animals. A pentobarbital based euthanasia solution will be utilized for euthanasia (e.g. Euthasol, 100 mg/kg, 1mL/10pounds body weight) while still under general anesthesia. The route of administration would either be IV through ear vein or femoral vein or intracardiac. Death will be confirmed with blood pressure-heart rate evaluation. V.5. Veterinary Care: V.5.1. Husbandry Considerations: Except as noted below, routine animal husbandry will be provided in accordance with LAM Husbandry SOPs for each species in this protocol. V.5.1.1. Study Room: Building Assigned by LAM Room V.5.1.2. Special Husbandry Provisions: Food Restriction: Pigs will receive no food after 1500 hrs on the day before the procedure, with fasting from at least 12 hrs to no more than 24 hrs prior to surgery. Fluid Restriction: None V.5.1.3. Exceptions: NA V.5.2. Veterinary Medical Care: USUHS Form 3206 – Revised February 2018 Previous versions are obsolete V.5.2.1. Routine Veterinary Medical Care: Animals will be observed and cared for by LAM personnel according to LAM SOPs. Health rounds are conducted by veterinary technicians and by animal husbandry personnel twice a day. Any animal deemed to be ill will be evaluated by the PI and/or a LAM Veterinarian and treated or euthanized as deemed appropriate. V.5.2.2. Emergency Veterinary Medical Care: All emergency, weekend, and holiday care is provided by two animal husbandry technicians, one or more veterinary technicians, and an on-call veterinarian. Essential husbandry procedures and health rounds are conducted by LAM personnel once daily during weekend and holidays. V.5.2.3 Environmental Enrichment: Except as indicated below, all animals on this protocol will be provided with routine environmental enrichment in accordance with LAM SOPs and IACUC Policies. Examples include balls, toys and food enrichment treats for large animal species. V.5.2.4. Enrichment Strategy: Prior to experimentation, all animals on this protocol will be provided with routine environmental enrichment in accordance with LAM SOPs and IACUC Policies. V.5.3.5. Enrichment Restrictions: None USUHS Form 3206 – Revised February 2018 Previous versions are obsolete VI. STUDY PERSONNEL QUALIFICATIONS AND TRAINING: STUDY PERSONNEL QUALIFICATIONS/TRAINING Protocol Activity Name Qualifications Specific Training USU IT Course/ IACUC/CITI Surgical procedures Todd Rasmussen MD Vascular surgeon USU PI training 04/09/18; 5/15/2018 ALAS Working with the IACUC; 5/15/2018 Introduction to Swine Surgical procedures Joseph White MD Vascular surgeon USU PI training 05/14/18; 5/16/2018 ALAS Working with the IACUC; 5/16/2018 Introduction to Swine Administration and tissue collections; laboratory assessments Thomas Davis PhD 35+ years in small and large animal model studies in DoD, academia and industry/biotech USU PI training 05/14/18; 5/15/2018 ALAS Working with the IACUC; 5/15/2018 Introduction to Swine Pre and Intraoperative care, assistance with surgical procedures John Mares B.S. Research Associate-III laboratory/Surgical Tech with an abundance of training in pre and post operative care in NHP and Swine models USU PI training 05/14/18; 5/21/2018 ALAS Working with the IACUC; 5/21/2018 Introduction to Swine Pre and Intraoperative care, assistance with surgical procedures Monet Schuler Research Associate-I Surgical Technician; wealth of training in pre and post operative emergency care for large animal USU PI training 05/14/18; 5/21/2018 ALAS Working with the IACUC; 5/21/2018 Introduction to Swine VII.BIOHAZARDS/SAFETY: USUHS Form 3206 – Revised February 2018 Previous versions are obsolete Biohazard concerns include: zoonotic disease control, lab safety (large animals will be used for this study), isoflurane exposure and waste gas safety, sharp instrument safety and any formalin exposure and safety measures. Isoflurane, Telazol, Ketamine, Xylazine, and Chlorprep will be stored and used in accordance with USU/LAM policy. All anesthetic gases will be properly scavenged. Occupational Health and Preventive Medicine guidelines and joint safety manual will be followed. All personnel participating in this protocol will be advised of the potential for zoonotic disease transmission. Investigators and Center for Laboratory Animal Medicine personnel will follow established standard USU/DoD Occupational Health and Preventive Medicine guidelines. “Occupational Health Program”. All personnel working with animals will wear protective clothing (scrubs, cap, mask, gloves, lab coat and shoe covers at a minimum). All disposable safety items are deposited in appropriate waste receptacles prior to departing the laboratory. All sharps are handled and disposed of in accordance with USU standard procedures. A. Zoonotic Disease: B. Safety Hazards: B. Isoflurane: C. Isoflurane Exposure: Safety procedures will be followed during use to include scavenging and properly insufflating the animal’s endotracheal tube cuff. D. Sharp Instruments: Syringes/needles used for injections will be disposed of in an appropriate sharps container. Lab staff are properly trained in the use of scalpels, syringes, and surgical instruments. E. Infectious Agents that do not cause Zoonoses: VIII. ENCLOSURES: IX. EXTRAMUAL COLLABORATION: If there are any collaborations with outside entities, please explain here. None.X. ASSURANCES: As the Principal Investigator on this protocol, I acknowledge my responsibilities and provide assurances for the following: A. Animal Use: The animals authorized for use in this protocol will be used only in the activities and in the manner described herein, unless a modification is specifically approved by the IACUC prior to its implementation. B. Duplication of Effort: I have made a reasonable, good faith effort to ensure that this protocol is not an unnecessary duplication of previous experiments. C. Statistical Assurance: I assure that I have consulted with an individual who is qualified to evaluate the statistical design or strategy of this proposal, and that the “minimum number of animals needed for scientific validity are used.” USUHS Form 3206 – Revised February 2018 Previous versions are obsolete D. Biohazard\Safety: I have taken into consideration and made the proper coordination regarding all applicable rules and regulations concerning radiation protection, biosafety, recombinant issues, and so forth, in the preparation of this protocol. E. Training: I verify that the personnel performing the animal procedures / manipulations / observations described in this protocol are technically competent and have been properly trained to ensure that no unnecessary pain or distress will be caused to the animals as a result of the procedures / manipulations. F. Training: I verify that I have attended the USUHS Investigator/Animal User Training Course. 21 May 2018 Principle Investigator Signature Date G. Training: The following personnel will attend the next USUHS Investigator/Animal User Training Course: 21 May 2018 Principle Investigator Signature Date H. Responsibility: I acknowledge the inherent moral, ethical and administrative obligations associated with the performance of this animal use protocol, and I assure that all individuals associated with this project will demonstrate a concern for the health, comfort, welfare, and well- being of the research animals. Additionally, I pledge to conduct this study in the spirit of the fourth "R" that the DOD has embraced, namely, "Responsibility" for implementing animal use alternatives where feasible and conducting humane and lawful research. 21 May 2018 Principle Investigator Signature Date I. Painful Procedure(s): I am conducting biomedical experiments which may potentially cause more than momentary or slight pain or distress to animals. This potential pain and/or distress WILL be relieved with the use of anesthetics, analgesics and/or tranquilizers. I have considered alternatives to such procedures; however, using the methods and sources described in the protocol, I have determined that alternative procedures are not available to accomplish the objectives of this proposed experiment. 21 May 2018 Principle Investigator Signature Date USUHS Form 3206 – Revised February 2018 Previous versions are obsolete XI. PROTOCOL ABSTRACT: A. Animal Protocol Number: Pending B. Animal Protocol Title: Partial resuscitative endovascular balloon occlusion of the aorta (PREBOA) characterization of targeted distal flow and permissive regional hypoperfusion in a porcine model (Sus scrofa domesticus) of hemorrhagic shock C. Principal Investigator: Joseph M. White, MD FACS MAJ MC USA Associate Program Director, Vascular Surgery Fellowship Assistant Professor of Surgery The Department of Surgery at Uniformed Services University of the Health Sciences and Walter Reed National Military Medical Center Office 301-295-8711 D. Performing Organization: Uniformed Services University of the Health Sciences E. Funding: USU F. Objective and Approach: The objectives of this study are two fold (1) to characterize distal aortic flow following PREBOA using the ER-REBOA, PREBOA-PRO and Coda LP (Cook Medical, Bloomington, IN) catheters in an established, translational, swine model of lethal non-compressible torso hemorrhage (NCTH) and (2) to identify the critical threshold at which Permissive Regional Hypoperfusion (PRH) allows for reduced perfusion pressure promoting clot stabilization (avoidance of hemorrhage), mitigates tissue ischemia and reperfusion injury, and dampens supraphysiologic central and cerebral pressures. Experiment 1: In a controlled prefixed bleed hemorrhagic shock model, evaluate and characterize distal aortic flow rate capabilities with the ER-REBOA, pREBOA-PRO and Coda LP (N= 8 swine per treatment arm; total 24 swine). Experiment 2: Following complete aortic occlusion and PRH in a swine traumatic liver amputation uncontrolled bleed hemorrhagic shock model (approximately 30% of the liver followed by 1.5 minutes of free hemorrhage), evaluate and characterize associated hemodynamics and metabolic/physiologic consequences of TDF at 50 mL/min (Grp 1), 150 mL/min (Grp 2), 300 mL/min (Grp 3) and 500 mL/min (Grp 4). N= 8 swine per treatment arm; total 32 swine. G. Indexing Terms (Descriptors): Partial resuscitative endovascular balloon occlusion of the aorta (PREBOA) Targeted Distal Flow (TDF) Permissive Regional Hypoperfusion (PRH) Porcine model (Sus scrofa domesticus) of hemorrhagic shock USUHS Form 3206 – Revised February 2018 Previous versions are obsolete References 1. Holcomb JB, McMullin NR, Pearse L, et al. Causes of death in US Special Operations Forces in the Global War on Terrorism. Ann Surgical 2007;245:986-991. 2. White JM, Stannard A, Burkhardt GE, et al. The epidemiology of vascular injury in the wars in Iraq and Afghanistan. Ann Surg. 2011 Jun;253(6):1184-9. 3. Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen- Gerdes L, Rasmussen TE, Butler FK, Kotwal RS, Holcomb JB, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH. Death on the battlefield (2001-2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012 Dec;73(6 Suppl 5):S431-7. 4. White JM, Cannon JW, Stannard A, Markov NP, Spencer JR, Rasmussen TE. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery. 2011 Sep;150(3):400-9. 5. DuBose JJ, Scalea T, et al. The AAST Prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) Registry: Data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg. 2016 Sep;81(3):409-19. 6. Williams TK, Neff LP, Johnson MA, Ferencz SA, Davidson AJ, Russo RM, Rasmussen TE. Extending resuscitative endovascular balloon occlusion of the aorta: Endovascular variable aortic control in a lethal model of hemorrhagic shock. J Trauma Acute Care Surg. 2016 Aug;81(2):294-301. USUHS Form 3206 – Revised February 2018 Previous versions are obsolete Appendix A Partial Resuscitative Endovascular Balloon Occlusion of the Aorta (P-REBOA) in a Pig Model (Sus scrota) Objectives: We investigated the hemodynamic and physiologic effects of partial Resuscitative Endovascular Balloon Occlusion of the Aorta ( REBOA .versus the current practiced complete REBOA (c- REBOA ) technique. Methods: Fifteen Yorkshire-cross swine were subjected to 25% total blood volume loss.They were randomized to either c- REBOA , p- REBOA , or no intervention. Aortic pressures, visceral arterial pressures, and serum makers of ischemia www.dtic.mil/docs/citations/AD1022767 - 221 KB - 2015-08-14 Partial Resuscitative Endovascular Balloon Occlusion of the Aorta (P-REBOA) in a Pig Model (Sus scrofa) with ongoing Resuscitation INTRODUCTION: Resuscitative Endovascular Balloon Occlusion of the Aorta ( REBOA ) is limited by the short duration of tolerable ischemia and resulting.reperfusion injury. P- REBOA relies on regional permissive hypo-perfusion to limit exsanguination while providing limited perfusion to distal tissue.beds. METHODS: An automated extracorporeal circuit was developed to perform P- REBOA . The circuit was used in a large animal model of highly lethal www.dtic.mil/docs/citations/AD1024014 - 452 KB - 2016-01-08 A Novel Fluoroscopy-free, Resuscitative Endovascular Aortic Balloon Occlusion System in a Model of Hemorrhagic Shock Michigan BACKGROUND: Resuscitative endovascular balloon occlusion of the aorta ( REBOA ) is a potentially lifesaving maneuver in the setting of.hemorrhagic shock. However, emergent use of REBOA is limited by existing technology, which requires large sheath arterial access and fluoroscopy- guided.balloon positioning. The objectives of this study were to describe a new, fluoroscopy-free REBOA system and to compare its efficacy to existing technology www.dtic.mil/docs/citations/ADA615139 - 1 MB - 2013-07-01 Partial Resuscitative Endovascular Balloon Occlusion of the Aorta (P-REBOA) in a Pig Model (Sus scrofa) Background: Partial REBOA (P- REBOA ) may permit longer periods of occlusion by allowing some degree of distal perfusion. However the ability of this.procedure to limit exsanguination is unclear. We evaluated the impact of P- REBOA on immediate survival in a highly lethal swine liver injury model.through hemodilution. Randomized swine received no intervention (control), P- REBOA , or complete REBOA (C- REBOA ). Central mean arterial pressure www.dtic.mil/docs/citations/AD1023092 - 173 KB - 2015-09-10 The Inflammatory Sequelae of Aortic Balloon Occlusion in Hemorrhagic Shock of the aorta REBOA Noncompressible torso hemorrhage Hemorrhagic shock Resuscitation a b s t r a c t Background.Resuscitative endovascular balloon occlusion of the aorta ( REBOA ) is a hemorrhage control and resuscitative adjunct that has been demonstrated to improve central perfusion during hemorrhagic shock.swine (Sus scrofa, weight 70 90 kg) underwent a 35% blood volume controlled hemorrhage followed by thoracic aortic balloon occlusion of 30 (30 REBOA) www.dtic.mil/docs/citations/ADA615153 - 1 MB - 2014-04-13 Aortic Balloon Occlusion is Effective in Controlling Pelvic Hemorrhage endo- vascular aortic balloon occlusion ( REBOA ) of the distal aorta in a porcine model of pelvic hemorrhage. Methods: Swine were entered into three.impregnated gauze (Combat Gauze) (CG, n 1⁄4 7), or REBOA (n 1⁄4 7). The protocol was repeated with a dilutional coagulopathy (CG-C, n 1⁄4 7, and REBOA -C, n.the REBOA and CG groups (822 415 mL/min USUHS Form 3206 – Revised February 2018 Previous versions are obsolete versus 11 13 and 0.2 0.4 mL/min respectively; P < 0.001). MAP following intervention (at 15 min) was the www.dtic.mil/docs/citations/ADA616881 - 408 KB - 2012-05-08 Assessing the Hemodynamic Effects of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) in Traumatic Cardiac Arrest When Closed Chest Compressions are Augmented by Directing the Area of Maximal Compression Over the Left Ventricle in a Swine Model (sus scrofa) Assessing the hemodynamic effects of Resuscitative Endovascular Balloon Occlusion of the Aorta ( REBOA ) in traumatic cardiac arrest when closed.survival (to 60 minutes), when performing chest compressions (CC) directly over the left ventricle, are maintained if REBOA is added to the www.dtic.mil/docs/citations/AD1017235 - 163 KB - 2016-09-15 Clinical Study of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for Severe Pelvic Fracture and Intra Abdominal Hemorrhagic Shock using Continuous Vital Signs AWARD NUMBER: W81XWH-15-1-0025 TITLE: Clinical Study of Resuscitative Endovascular Balloon Occlusion of the Aorta ( REBOA ) for Severe Pelvic. REBOA ) for Severe Pelvic Fracture & Intra- Abdominal Hemorrhagic Shock using Continuous Vital Signs 5c. PROGRAM ELEMENT NUMBER 6. AUTHOR(S) 5d.sites. Resuscitative balloon occlusion of the aorta ( REBOA ) has been clinically demonstrated to stop bleeding below the diaphragm. It has the potential www.dtic.mil/docs/citations/AD1033989 - 2 MB - 2016-03-01 Technique Development for a Polytrauma Model to Study Partial Resuscitative Endovascular Balloon Occlusion of the Aorta (P-REBOA) in Swine (Sus scrofa) p- reboa (partial resuscitative endovascular balloon occlusion of the aorta) www.dtic.mil/docs/citations/AD1024018 - 429 KB - 2016-06-01 Clinical Study of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for Severe Pelvic Fracture and Intra Abdominal Hemorrhagic Shock using Continuous Vital Signs AWARD NUMBER: W81XWH-15-1-0025 TITLE: Clinical Study of Resuscitative Endovascular Balloon Occlusion of the Aorta ( REBOA ) for Severe Pelvic.Hemorrhagic Shock 5b. GRANT NUMBER W81XWH-15-1-0025 Clinical Study of Resuscitative Endovascular Balloon Occlusion of the Aorta ( REBOA ) for Severe.Placed REBOA Appendix B: Brief Clinical Summary and Review of Data Capture 28 Process: Continuous Vital Signs (VS) and Videography Appendix C www.dtic.mil/docs/citations/AD1043372 - 2 MB - 2017-11-01 PDF The Effect Of Supraphysiologic Blood Pressure on Traumatic Brain Injury and Proximal Tissue Beds During Resuscitative Balloon Occlusion of the Aorta and Variable Aortic Control in a Porcine Model (Sus scrofa) of Polytrauma. Mandatory) The Effect of REBOA , Partial Aortic Occlusion and Aggressive Blood Transfusion on Traumatic Brain Injury in a Swine Polytrauma Model.Objectives: Despite clinical reports of poor outcomes, the degree to which REBOA exacerbates traumatic brain injury (TBI) is not known. We hypothesized that.combined effects of increased proximal mean arterial pressure (pMAP), carotid blood flow (Qcarotid), and intracranial pressure (ICP) from REBOA would www.dtic.mil/docs/citations/AD1032867 - 254 KB - 2017-04-27 PDF Physiologic Tolerance of Descending Thoracic Aortic Balloon Occlusion in a Swine Model of Hemorrhagic Shock REBOA ) is an emerging technique in trauma; however, the physiological sequelae are un- quantified. The objectives of this study are to characterize the.burden of reperfusion and organ USUHS Form 3206 – Revised February 2018 Previous versions are obsolete dysfunction of REBOA incurred during 30 or 90 minutes of class IV shock in a survivable porcine model of hemorrhage.Methods: Following the induction of shock, animals were randomized into 4 groups (n=6): 30 minutes of shock alone (30-Shock) or REBOA (30- REBOA ) and www.dtic.mil/docs/citations/ADA568504 - 1 MB - 2013-01-10 PDF Resuscitative Endovascular Balloon Occlusion of the Aorta Improves Survival in Lethal Hemorrhage Resuscitative Endovascular Balloon Occlusion of the Aorta ( REBOA ) using generic balloon catheters placed into the descending aorta under fluoroscopy has.been used to treat hemorrhagic shock in animal models. We tested a new non-image guided 7F ER- REBOA catheter (Pryor Medical Arvada, CO) for its.potential to improve survival in a 100% lethal model of hemorrhagic shock. We hypothesized that ER- REBOA , placed without fluoroscopic guidance, improves www.dtic.mil/docs/citations/ADA614875 - 55 KB - 2014-01-01 PDF The Effect of Infrarenal Aortic Balloon Occlusion on Weaning from Supraceliac Aortic Balloon Occlusion in a Porcine Model (Sus scrofa) of Hemorrhagic Shock Objectives: We sought to determine whether stepwise reperfusion after supraceliac (Zone-1) REBOA by transitioning to infrarenal (Zone-3) occlusion.hemorrhage of 25 blood volume, 45 minutes of Zone-1 REBOA , then resuscitation with shed blood. Critical care began with deflation of the Zone-1 balloon in.all animals, and continued for six hours. Half of the animals were randomly assigned to Zone-3 REBOA for an additional 45 minutes following Zone-1 www.dtic.mil/docs/citations/AD1035356 - 755 KB - 2017-06-15 PDF Assessing Hemorrhage Severity with Continuous Automatic Heart-Rate-Complexity Monitoring in Swine transfusion of shed blood (TSB, n=7); or endovascular balloon occlusion of the aorta ( REBOA ) (Pryor Medical, Arvada, CO) for up to 60 minutes followed.by TSB (n=21). Epinephrine boluses were given if mean arterial pressure (MAP) < 40 mmHg in the TSB and REBOA groups. After resuscitative.7 in group TSB, and 1/21 in group REBOA . SampEn and MSE decreased with hemorrhage and increased after interventions (TSB or REBOA +TSB). www.dtic.mil/docs/citations/ADA621471 - 56 KB - 2014-12-02 PDF Endovascular Perfusion Augmentation for Critical Care (EPACC) as a Resuscitative Adjunct ina Swine (Sus scrofa) Polytrauma Model of Ischemia Reperfusion Injury. Florida in January. This work has resulted in one manuscript entitled Location Is Everything: The Hemodynamic Effects of REBOA in Zone 1 versus Zone 3 of.wounded soldiers in hemorrhagic shock when REBOA is going to be used. 12. PROTOCOL OUTCOME SUMMARY: (Please provide, in "ABSTRACT" format, a summary.Endovascular Balloon Occlusion of the Aorta ( REBOA ) is an emerging technology to augment proximal blood pressure during the resuscitation of patients with non www.dtic.mil/docs/citations/AD1044086 - 874 KB - 2017-12-21 PDF The Effect of Hypothermia on Prolonged Distal Aortic Balloon Occlusion in a Porcine Model (Sus scrofa) of Hemorrhage normothermia followed by 4 hours of zone III REBOA , resuscitation with shed blood, and 3 hours of critical care. Physiologic parameters were continuously.Conclusion: External cooling during prolonged zone III REBOA decreased ischemic muscle injury and resulted in lower compartment pressures following www.dtic.mil/docs/citations/AD1038226 - 657 KB - 2017-06- 12 PDF USUHS Form 3206 – Revised February 2018 Previous versions are obsolete Prospective Evaluation of the Correlation Between Torso Height and Aortic Anatomy in Respect of a Fluoroscopy Free Aortic Balloon Occlusion System endovascular balloon occlusion of the aorta ( REBOA ) provides inflow control and afterload support to patients with circulatory collapse from hemorrhage.7 It can.deterioration, or as a substitute to open cross- clamping in the moribund patient.8 REBOA is de- signed as a proactive maneuver, which can be in- serted in.aortic balloon occlusion during endovascular aneurysm repair,12,13 many of these constrains have been overcome. The use of REBOA in traumatic hemorrhagic www.dtic.mil/docs/citations/ADA614718 - 547 KB - 2014-06-01 PDF Endovascular Therapy in Trauma of the aorta ( REBOA ) The most common cause of death from aortic trauma remains hemorrhagic shock compounded by ongoing coagulopathy, thus early.with REBOA [37–40]. Descriptions of its use for trauma are few [41–43], but may be useful for control of non- compressible torso hemorrhage in the.these areas, but the potential exists to train trauma surgeons in this technique. The use of REBOA to obtain proximal control at the level of the www.dtic.mil/docs/citations/ADA620818 - 528 KB - 2014-11-23 PDF Technique Refinement and Validation of Variable Aortic Occlusion via Extracorporeal Flow Circuit in a Pig Model (Sus scrofa) of Uncontrolled Hemorrhage with Subsequent Resuscitation and Critical Care Rasmussen TE. Extending REBOA : Endovascular Variable Aortic Control (EVAC) in a Lethal Model of Hemorrhagic Shock. The Journal of Trauma and Acute Care www.dtic.mil/docs/citations/AD1022574 - 698 KB - 2016-07-24 Morphometric Analysis of Torso Arterial Anatomy with Implications for Resuscitative Aortic Occlusion balloon occlusion of the aorta ( REBOA ) as an adjunct for hemorrhagic shock. J Trauma. 2011;71:1869Y1872. 5. Sierink JC, Saltzherr TP, Beenen LFM, Luitse JSK www.dtic.mil/docs/citations/ADA614948 - 4 MB - 2013-08-01 PREBOA Pre-clinical Experience (large animal models) White JM, Cannon JW, Stannard A, Markov NP, Spencer JR, Rasmussen TE. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery. 2011 Sep;150(3):400-9. doi: 10.1016/j.surg.2011.06.010. The objective of this study is to characterize resuscitative aortic balloon occlusion (BO) compared to thoracotomy with aortic clamping in a model of hemorrhagic shock. A total of 18 swine (3 groups; 6 animals/group) were used in this study. Swine in class IV shock underwent no aortic occlusion (NO), thoracotomy and clamp occlusion (CO), or endovascular BO. Animals in the NO group underwent direct placement of a temporary vascular shunt (TVS) at the injury site, whereas animals in the CO and BO groups underwent aortic occlusion before TVS placement. Hemodynamic and physiologic measures were collected.The central aortic pressure, carotid blood flow and brain oxygenation as measured by oximetry increased in the CO and BO groups compared to the NO group (P < .05). During resuscitation, the BO group was less acidotic than the CO group (pH,7.35 vs 7.24; P < .05) with a lower serum lactate level (4.27 vs 6.55; P < .05) and pCO2 level (43.5 vs 49.9; P < .05). During resuscitation, the BO group required less fluid (667 mL vs 2,166 mL; P < .05) and norepinephrine (0 mcg vs 52.1 mcg; P < .05) than the CO group. Resuscitative aortic BO increases central perfusion pressures with less physiologic disturbance than thoracotomy with aortic clamping in a model of hemorrhagic shock. USUHS Form 3206 – Revised February 2018 Previous versions are obsolete Endovascular BO of the aorta should be explored further as an option in the management of noncompressible torso hemorrhage (NCTH). Davidson AJ, Russo RM, Ferencz SE, Cannon JW, Rasmussen TE, Neff LP, Johnson MA, Williams TK. Incremental balloon deflation following complete resuscitative endovascular balloon occlusion of the aorta results in steep inflection of flow and rapid reperfusion in a large animal model of hemorrhagic shock. J Trauma Acute Care Surg. 2017 Jul;83(1):139-143. doi: 10.1097/TA.0000000000001502. Study completed in 2016. The objective of this study was to characterize the hemodynamic effect of incremental balloon deflation following sustained REBOA, particularly with respect to mean arterial pressure (MAP) and aortic flow. Hemorrhage shock was simulated (25% total estimate blood volume). Next, complete aortic occlusion was initiated with ER-REBOA for 60 minutes. This was followed by incremental balloon deflation of 0.5cc every 30 seconds. Physiologic parameters and aortic flow measurements were collected. Rapid increase in aortic flow during single-balloon deflation was observed in all animals (664-1241 mL/min MAX). Key observations: (1) a large proportion of aortic flow returned during a discrete time point (i.e., deflation step); (2) the precise onset of this rapid return of flow was variable and unpredictable across animals; (3) this rapid reintroduction of aortic flow corresponded with a significant drop in MAP despite ongoing aggressive blood resuscitation. Johnson MA, Davidson AJ, Russo RM, Ferencz SE, Gotlib O, Rasmussen TE, Neff LP, Williams TK. Small changes, big effects: The hemodynamics of partial and complete aortic occlusion to inform next generation resuscitation techniques and technologies. J Trauma Acute Care Surg. 2017 Jun;82(6):1106-1111. doi: 10.1097/TA.0000000000001446. The study hypothesized that proximal blood pressure would be an inaccurate predictor of distal aortic flow but that distal mean arterial blood pressure (MAP) would provide a more accurate estimate of aortic flow at various levels of hemorrhage following complete aortic occlusion. The aim of the study was to examine the relationship between blood pressure and aortic flow at varying degrees of occlusion, across iterative levels of hemorrhage. The seven animals used in this experiment had no significant differences in baseline physiology. Following full clamp release at both 10% and 20% hemorrhage, aortic flow was markedly increased relative to baseline flow (10%: 1,599 ± 785 mL/min, p < 0.01; 20%: 1,070 ± 396 mL/min, p < 0.01), but increased flow was not evident after 30% and 40% hemorrhage (30%: 337 ± 490 mL/min, p = 0.12; 40%: −83 ± 256 mL/min, p = 0.42). During aortic occlusion, proximal MAP increased for all levels of hemorrhage when compared with baseline. During occlusion, a distal MAP was present at all levels of hemorrhage. The average distal MAP during occlusion was smaller after 40% hemorrhage when compared with 10% hemorrhage (6.5 ± 2.6 mmHg vs 10.0 ± 1.7 mmHg, Fig. 3C, p = 0.03). During clamp release, aortic flow was linearly related to distal MAP across all levels of hemorrhage. Aortic flow increased at a rate of 30.7 ± 10.1 mL/min (40% hemorrhage) to 50.5 ± 10.8 mL/min (10% hemorrhage) for every increase of 1 mm Hg in MAP, with no statistical differences between groups (p > 0.05). Aortic flow was not linearly related to proximal MAP at any level of hemorrhage. This study described the relationship between proximal blood pressure, distal blood pressure, and distal aortic blood flow during aortic occlusion and reperfusion at varying shock states. The study demonstrated that supraphysiologic blood pressure occurs during occlusion in Classes I and II shock, which has the potential to exacerbate injuries to the brain and create increased stress on the cardiopulmonary system. Furthermore, the study demonstrated that aortic hyperemia occurs during reperfusion from complete occlusion in Classes I and II shock. Finally, the study demonstrated that aortic blood USUHS Form 3206 – Revised February 2018 Previous versions are obsolete flow has a linear relationship with distal blood pressure during reperfusion, a critical concept that can be used to help transition from complete to pREBOA. Russo RM, Neff LP, Lamb CM, Cannon JW, Galante JM, Clement NF, Grayson JK, Williams TK. Partial Resuscitative Endovascular Balloon Occlusion of the Aorta in Swine Model of Hemorrhagic Shock. J Am Coll Surg. 2016 Aug;223(2):359-68. doi: 10.1016/j.jamcollsurg.2016.04.037. Epub 2016 Apr 29. The physiologic impact of P-REBOA for sustained therapy in hemorrhagic shock has not been fully characterized. In an effort to test this effect, we hypothesized P-REBOA would preserve proximal aortic mean arterial pressure (MAP) closer to normal physiologic levels and concurrently reduce distal ischemia and systemic metabolic injury compared with C-REBOA in a porcine hemorrhagic shock model. In this study, we have demonstrated that P-REBOA maintained proximal MAP at more physiologically normal levels during intervention and after balloon deflation, and avoided the hemodynamic extremes seen with C-REBOA. Partial REBOA also simultaneously maintained enough distal perfusion to minimize organ ischemia and the systemic burden of ischemia reperfusion injury compared with C-REBOA. Maintaining proximal MAP within a normal physiologic range with P-REBOA can reduce the incidence of cardiac dysfunction, cerebral edema, and respiratory failure compared with sustained aortic occlusion. Partial REBOA can also offer a way to extend the duration of intervention beyond what is currently possible with C-REBOA. Russo RM, Williams TK, Grayson JK, Lamb CM, Cannon JW, Clement NF, Galante JM, Neff LP. Extending the golden hour: Partial resuscitative endovascular balloon occlusion of the aorta in a highly lethal swine liver injury model. J Trauma Acute Care Surg. 2016 Mar;80(3):372-8; discussion 378-80. doi: 10.1097/TA.0000000000000940. The objective of this study was to examine the feasibility and effectiveness of sustained P- REBOA compared with complete REBOA (CREBOA) in a porcine model of lethal hemorrhagic shock. In addition, this investigation aimed to characterize the impact of P-REBOA compared with C-REBOA on cMAP, distal hemorrhage, and markers of ischemia-reperfusion injury. Procedural blood losswas quantified, and additional blood was removed to standardize preinjury blood loss at 10% of the total circulating blood volume (6.6 mL/kg) over 5 minutes. The liver was marked along the planned transection plane, 2 cm to the left of Cantlie's line, to provide amputation of approximately 80% of the left lateral lobe of the liver and 40% of the left medial lobe of the liver (approximately 30% of the total liver volume) similar to previous descriptions. The swine were then assigned via a block randomization scheme to no intervention (controls), P-REBOA, or C-REBOA. In this study of highly lethal uncontrolled hemorrhage in swine, P- REBOA proved effective at maintaining a reliable and reproducible aortic pressure gradient over time, despite ongoing hemorrhage. P-REBOA increased survival time beyond the golden hour while maintaining physiologic cMAP and carotid blood flow. This preservation of baseline proximal pressure and flow may theoretically reduce injury to tissue beds above the point of aortic occlusion. USUHS Form 3206 – Revised February 2018 Previous versions are obsolete Appendix B: PI NAME: COL Todd Rasmussen, MD; USAF MC PROTCOL NUMBER: DRUGS AND CONTROLLED SUBSTANCES Please list ALL drugs and controlled substances that will be used under this protocol, indicating the DEA Schedule if known. Provide both the Approved Name and the Proprietary Name of each drug if known. This list, once approved, will be used by the IACUC and the USU Pharmacy to determine which drugs and controlled substances can be supplied to the Principal Investigator. The Pharmacy will not dispense to the PI any drug that is not included in this list. Drug Name DEA Schedule (I, II, III, or IV) (Please give both approved and (if known) Proprietary Names if possible) _____________________________ _________________________ Issued protocol number SUR-19-965. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
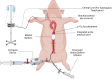
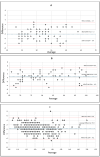

References
-
- Death on the battlefield (2001-2011): implications for the future of combat casualty care. Eastridge BJ, Mabry RL, Seguin P, et al. J Trauma Acute Care Surg. 2012;73:0–7. - PubMed
-
- Field and en route resuscitative endovascular occlusion of the aorta: a feasible military reality? Reva VA, Hörer TM, Makhnovskiy AI, Sokhranov MV, Samokhvalov IM, DuBose JJ. J Trauma Acute Care Surg. 2017;83:0–6. - PubMed
-
- A modern case series of resuscitative endovascular balloon occlusion of the aorta (REBOA) in an out-of-hospital, combat casualty care setting. Manley JD, Mitchell BJ, DuBose JJ, Rasmussen TE. J Spec Oper Med. 2017;17:1–8. - PubMed
-
- Recent advances in austere combat surgery: use of aortic balloon occlusion as well as blood challenges by special operations medical forces in recent combat operations. Northern DM, Manley JD, Lyon R, et al. J Trauma Acute Care Surg. 2018;85:0. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
