Efficacy of Oral Nanoparticle-Encapsulated Insulin in Reducing Oxidative Stress and Enhancing Tissue Integrity in a Diabetic Rat Model
- PMID: 39493274
- PMCID: PMC11529609
- DOI: 10.2147/IJN.S468756
Efficacy of Oral Nanoparticle-Encapsulated Insulin in Reducing Oxidative Stress and Enhancing Tissue Integrity in a Diabetic Rat Model
Abstract
Introduction: Diabetes mellitus, a chronic metabolic disorder, leads to systemic organ damage characterized by oxidative stress and structural alterations, contributing to increased morbidity and mortality. Traditional subcutaneous insulin therapy, while managing hyperglycemia, often falls short in addressing the oxidative damage and preventing organ-specific complications. This study evaluates the therapeutic efficacy of a novel oral nanoparticle-mediated insulin (nCOF/Insulin) against these diabetes-induced changes, comparing it with traditional subcutaneous insulin in a streptozotocin (STZ)-induced diabetic rat model.
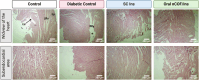
Methods: We induced diabetes in Wistar rats, dividing them into four groups: standard control, diabetic control, diabetic treated with subcutaneous insulin, and diabetic treated with oral nanoparticle-mediated insulin (nCOF/Insulin). Assessments included organ and body weights, histopathological examinations, and oxidative stress markers (MDA and PCOs) across various organs, including the brain, muscle, intestine, spleen, heart, liver, kidney, and adrenal glands. Additionally, we evaluated antioxidant parameters (GSH and catalase) and conducted immunohistochemical analysis of E-cadherin to assess intestinal integrity.
Results: Our findings reveal that STZ-induced diabetes significantly impacts organ health, with subcutaneous insulin providing limited mitigation and, in some cases, exacerbating oxidative stress. Conversely, oral nCOF/Insulin treatment effectively restored organ and body weights, reduced oxidative stress markers, and mitigated histological damage. This suggests that oral nCOF/Insulin not only offers superior glycemic control but also addresses the underlying oxidative stress.
Conclusion: nCOF/Insulin emerges as a promising treatment for diabetes, with the potential to improve patient quality of life by ameliorating oxidative stress and preventing organ-specific complications. This study underscores the need for further investigation into the long-term effects and mechanisms of action of oral nCOF/Insulin, aiming to revolutionize diabetes management and treatment strategies.
Keywords: diabetes mellitus; diabetic complications; oral nanoparticle-mediated insulin; organ toxicity; oxidative stress.
© 2024 Kaddour et al.
Conflict of interest statement
The authors declare that they have no competing interests in this work.
Figures








References
-
- World health organization. Class Diabetes Mellitus. 2019.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

