SEETrials: Leveraging large language models for safety and efficacy extraction in oncology clinical trials
- PMID: 39493413
- PMCID: PMC11530223
- DOI: 10.1016/j.imu.2024.101589
SEETrials: Leveraging large language models for safety and efficacy extraction in oncology clinical trials
Abstract
Background: Initial insights into oncology clinical trial outcomes are often gleaned manually from conference abstracts. We aimed to develop an automated system to extract safety and efficacy information from study abstracts with high precision and fine granularity, transforming them into computable data for timely clinical decision-making.
Methods: We collected clinical trial abstracts from key conferences and PubMed (2012-2023). The SEETrials system was developed with three modules: preprocessing, prompt engineering with knowledge ingestion, and postprocessing. We evaluated the system's performance qualitatively and quantitatively and assessed its generalizability across different cancer types- multiple myeloma (MM), breast, lung, lymphoma, and leukemia. Furthermore, the efficacy and safety of innovative therapies, including CAR-T, bispecific antibodies, and antibody-drug conjugates (ADC), in MM were analyzed across a large scale of clinical trial studies.
Results: SEETrials achieved high precision (0.964), recall (sensitivity) (0.988), and F1 score (0.974) across 70 data elements present in the MM trial studies Generalizability tests on four additional cancers yielded precision, recall, and F1 scores within the 0.979-0.992 range. Variation in the distribution of safety and efficacy-related entities was observed across diverse therapies, with certain adverse events more common in specific treatments. Comparative performance analysis using overall response rate (ORR) and complete response (CR) highlighted differences among therapies: CAR-T (ORR: 88 %, 95 % CI: 84-92 %; CR: 95 %, 95 % CI: 53-66 %), bispecific antibodies (ORR: 64 %, 95 % CI: 55-73 %; CR: 27 %, 95 % CI: 16-37 %), and ADC (ORR: 51 %, 95 % CI: 37-65 %; CR: 26 %, 95 % CI: 1-51 %). Notable study heterogeneity was identified (>75 % I 2 heterogeneity index scores) across several outcome entities analyzed within therapy subgroups.
Conclusion: SEETrials demonstrated highly accurate data extraction and versatility across different therapeutics and various cancer domains. Its automated processing of large datasets facilitates nuanced data comparisons, promoting the swift and effective dissemination of clinical insights.
Keywords: Automated safety and efficacy extraction; Conference abstracts; GPT-4; Large language models; Large scale analysis; Oncology clinical trial.
Conflict of interest statement
KL, HP, SD, LH, NO, FM, JW, and XW are currently employees of IMO Health Inc. JLW reports funding from NCI/NIH, related to the work, funding from AACR and Brown Physicians Incorporated, consulting from Westat, and ownership of HemOnc.org LLC, outside the scope of the work. No other conflict of interest.Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
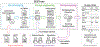





Update of
-
SEETrials: Leveraging Large Language Models for Safety and Efficacy Extraction in Oncology Clinical Trials.medRxiv [Preprint]. 2024 May 13:2024.01.18.24301502. doi: 10.1101/2024.01.18.24301502. medRxiv. 2024. Update in: Inform Med Unlocked. 2024;50:101589. doi: 10.1016/j.imu.2024.101589. PMID: 38798420 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
