Patients with Growth-Related Disorders and Caregivers Prefer the Somapacitan Device to the Somatrogon Device: Results from a Randomized Crossover Study Assessing Device Preference and Ease of Use Following Simulated Injections
- PMID: 39493440
- PMCID: PMC11531717
- DOI: 10.2147/MDER.S484354
Patients with Growth-Related Disorders and Caregivers Prefer the Somapacitan Device to the Somatrogon Device: Results from a Randomized Crossover Study Assessing Device Preference and Ease of Use Following Simulated Injections
Abstract
Purpose: Adherence to growth hormone treatment is known to affect growth outcomes. Both device preference and ease of use have been shown to affect treatment adherence. In this study, we assessed device preference and ease of use with two long-acting growth hormones, somapacitan (Sogroya®, Novo Nordisk A/S) and somatrogon (Ngenla®, Pfizer).
Patients and methods: In a randomized, crossover study conducted between September 20 and November 2, 2023, we recruited 33 adolescents with a growth-related disorder, and 37 caregivers, at six locations in the United States. Each participant was trained in the use of both devices and asked to perform a simulated injection. Device training time, preparation and injection time, and injection completeness were recorded. Participants also completed the Device Handling and Preference Questionnaire (DHPAQ) to indicate their device preference and ease of use opinions. Following conclusion of the "standard" visit, 10 adolescents and 10 caregivers were randomly selected to participate in a sub-study to validate the relevance, comprehensiveness, and comprehension of the DHPAQ.
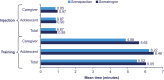
Results: The majority of participants (84.3%; 95% confidence interval [CI]: 74;92) preferred the somapacitan device to the somatrogon device (p < 0.0001). Almost all (98.6%; 95% CI: 92;100) participants answered that the somapacitan device was easy or very easy to use, while three-quarters (74.3%; 95% CI: 62;84) answered the same for the somatrogon device. Average training and injection times were lower for the somapacitan device than for the somatrogon device. Also, more patients successfully completed the injection with the somapacitan device than with the somatrogon device (97.1% vs 92.9%). Cognitive debriefing interviews indicated the DHPAQ was relevant, comprehensive, and fully comprehended.
Conclusion: The somapacitan device was preferred to the somatrogon device by a majority of participants. More participants considered the somapacitan device to be easy or very easy to use than the somatrogon device.
Keywords: device preference; ease of use; growth hormone deficiency; long-acting growth hormone; somapacitan; somatrogon.
Plain language summary
• Children who grow slower in height than their peers often need growth hormone replacement treatment to reach normal height. This treatment involves daily or weekly injections for several years. Injecting treatment regularly is important for it to work properly, but the schedule can be difficult to adhere to. • From previous research, we know injection devices can influence adherence to treatment. Researchers in this study aimed to compare two different pen injectors (once-weekly medicines called somapacitan and somatrogon) to see which one patients and caregivers prefer and find easier to use. • This USA-based study included 33 adolescents with growth problems who injected themselves, and 37 caregivers of younger patients. During one visit, they were trained in how to use each device, observed giving a practice injection, and asked to fill in a questionnaire with their experience. Half of the participants did this with the somapacitan pen injector first and the somatrogon one second, while the other half followed the opposite order. • The researchers found that 84.3% of participants preferred the somapacitan pen‑injector, while 12.9% preferred the somatrogon pen-injector, and this difference was statistically significant. Almost all (98.6%) participants found the somapacitan pen‑injector easy or very easy to use, while three-quarters (74.3%) answered the same for somatrogon. On average, the participants learned to use the somapacitan device faster and more correctly than the somatrogon device. • In conclusion, given the device preference and ease of use, patients may be more likely to adhere to treatment with somapacitan compared with those receiving somatrogon.
© 2024 Akhtar et al.
Conflict of interest statement
Shahid Akhtar, Birgitte Berg, Johan Medina, Sophie Hamilton, Nicky Kelepouris, Jesper Skov Neergaard, Claus Sværke, Gitte Ter-Borch, and Niklas Kahr Rasmussen are employees of, and hold stocks in, Novo Nordisk. Maya Gonczi and Emily Hildebrand are employees of Research Collective, which received financial compensation from Novo Nordisk for performing the research reported in the manuscript. The authors report no other conflicts of interest in this work.
Figures
References
LinkOut - more resources
Full Text Sources