Human papillomavirus infection affects the immune microenvironment and antigen presentation in penile cancer
- PMID: 39493451
- PMCID: PMC11527599
- DOI: 10.3389/fonc.2024.1463445
Human papillomavirus infection affects the immune microenvironment and antigen presentation in penile cancer
Abstract
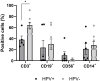
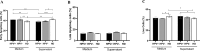
Penile squamous cell carcinoma (PSCC) is a largely neglected condition, predominantly affecting underdeveloped regions, and is associated with risk factors such as low socioeconomic status, phimosis, and human papillomavirus (HPV) infection. Unlike other urogenital cancers, its pathophysiology and therapeutic targets remain poorly understood, particularly regarding the immune response to the tumor microenvironment. This study aims to investigate immune cell infiltration profiles, dendritic cell maturation, and lymphocyte apoptosis in both HPV-positive and HPV-negative PSCC. Clinical and histopathological data, along with peripheral blood and tumor tissue samples, were collected from 30 patients (66.6% were HPV-positive and 33.3% HPV-negative), with an additional 19 healthy donors serving as controls. Tumor-infiltrating immune cells were analyzed following enzymatic digestion of tumor tissue, enabling detailed phenotypic characterization. A simulated tumor microenvironment was created using supernatants derived from primary cultures of HPV-positive PSCC tumors. Peripheral blood mononuclear cells were isolated and differentiated into dendritic cells (Mo-DCs) for further phenotyping and lymphoproliferation assays. Lymphocytes from healthy donors and patients were exposed to tumor culture supernatants to evaluate apoptosis induced by the tumor microenvironment. Results showed that HPV-positive tumors exhibited lower T lymphocyte frequencies compared to HPV-negative tumors. Additionally, patients infected with high-risk HPV demonstrated reduced maturation rates of Mo-DCs and decreased expression of co-stimulatory molecules on these cells compared to healthy donors. Furthermore, Mo-DCs from hrHPV-positive patients showed impaired lymphoproliferation capacity relative to controls, while HPV-negative patients exhibited a trend towards reduced lymphoproliferative ability. Regarding the simulated tumor microenvironment, lymphocytes from healthy donors underwent apoptosis, contrasting with patients' lymphocytes, which showed increased viability when cultured with tumor supernatants. These results underscore the impact of HPV infection on T lymphocyte infiltration, Mo-DC maturation, and lymphocyte survival in PSCC, offering critical insights for advancing our understanding of the tumor microenvironment and guiding the development of immunotherapy strategies.
Keywords: HPV-related cancer; cancer immunomodulation; costimulatory molecules; dendritic cells; urological carcinoma.
Copyright © 2024 Guimarães, Vale, Rocha, Butarelli, da Silva, Deus, Nogueira, Coelho, Pereira and Azevedo-Santos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Progressive T cell exhaustion and predominance of aging tissue associated macrophages with advancing disease stage in penile squamous cell carcinoma.Sci Rep. 2025 Mar 5;15(1):7703. doi: 10.1038/s41598-025-89760-0. Sci Rep. 2025. PMID: 40044748 Free PMC article.
-
Comprehensive genomic profiling of penile squamous cell carcinoma and the impact of human papillomavirus status on immune-checkpoint inhibitor-related biomarkers.Cancer. 2023 Dec 15;129(24):3884-3893. doi: 10.1002/cncr.34982. Epub 2023 Aug 11. Cancer. 2023. PMID: 37565840
-
The effects of HIV and oncogenic human papillomavirus on the tumor immune microenvironment of penile squamous cell carcinoma.PLoS One. 2024 May 1;19(5):e0300729. doi: 10.1371/journal.pone.0300729. eCollection 2024. PLoS One. 2024. PMID: 38691575 Free PMC article.
-
Immune landscape and immunotherapy for penile cancer.Front Immunol. 2022 Nov 29;13:1055235. doi: 10.3389/fimmu.2022.1055235. eCollection 2022. Front Immunol. 2022. PMID: 36524123 Free PMC article. Review.
-
A comprehensive review of current knowledge on penile squamous cell carcinoma.Front Oncol. 2024 May 22;14:1375882. doi: 10.3389/fonc.2024.1375882. eCollection 2024. Front Oncol. 2024. PMID: 38841163 Free PMC article. Review.
Cited by
-
Inflammation in Penile Squamous Cell Carcinoma: A Comprehensive Review.Int J Mol Sci. 2025 Mar 19;26(6):2785. doi: 10.3390/ijms26062785. Int J Mol Sci. 2025. PMID: 40141426 Free PMC article. Review.
-
Smoking and presence of human papillomavirus correlates with lymphocyte density in the stroma of penile squamous cell carcinoma.Front Oncol. 2025 Mar 31;15:1568764. doi: 10.3389/fonc.2025.1568764. eCollection 2025. Front Oncol. 2025. PMID: 40231254 Free PMC article.
-
A review of the carcinogenic potential of human papillomavirus (HPV) in urological cancers.Virol J. 2025 Feb 28;22(1):53. doi: 10.1186/s12985-025-02682-1. Virol J. 2025. PMID: 40022189 Free PMC article. Review.
References
-
- Macedo J, Silva E, Nogueira L, Coelho R, da Silva J, Dos Santos A, et al. . Genomic profiling reveals the pivotal role of hrHPV driving copy number and gene expression alterations, including mRNA downregulation of TP53 and RB1 in penile cancer. Mol Carcinog. (2020) 59:604–17. doi: 10.1002/mc.23185 - DOI - PubMed
LinkOut - more resources
Full Text Sources

