Beta-Band Cortico-Muscular Phase Coherence in Hemiparetic Stroke
- PMID: 39493553
- PMCID: PMC11526780
- DOI: 10.1016/j.bspc.2024.106719
Beta-Band Cortico-Muscular Phase Coherence in Hemiparetic Stroke
Abstract
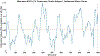
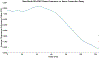
Following a stroke, compensation for the loss of ipsilesional corticospinal and corticobulbar projections, results in increased reliance on contralesional motor pathways during paretic arm movement. Better understanding outcomes of post-stroke contralesional cortical adaptation outcomes may benefit more targeted post-stroke motor rehabilitation interventions. This proof-of-concept study involves eight healthy controls and ten post-stroke participants. Electroencephalographic (EEG) and deltoid electromyographic (EMG) data were collected during an upper-limb task. Phase coupling between beta-band motor cortex EEG and deltoid EMG was assessed using the Multi-Phase Locking Value (M-PLV) method. Different from classic cortico-muscular coherence, M-PLV allows for the calculation of dynamic phase coherence and delays, and is not affected by the non-stationary nature of EEG/EMG signals. Nerve conduction delay from the contralateral motor cortex to the deltoid muscle of the paretic arm was estimated. Our results show the ipsilateral (contralesional) motor cortex beta-band phase coherence behavior is altered in stroke participants, with significant differences in ipsilateral EEG-EMG coherence values, ipsilateral time course percentage above the significance threshold, and ipsilateral time course area above the significance threshold. M-PLV phase coherence analysis provides evidence for post-stroke contralesional motor adaptation, highlighting its increased role in the paretic shoulder abduction task. Nerve conduction delay between the motor cortices and deltoid muscle is significantly higher in stroke participants. Beta-band M-PLV phase coherence analysis shows greater phase-coherence distribution convergence between the ipsilateral (contralesional) and contralateral (ipsilesional) motor cortices in stroke participants, which is interpretable as evidence of maladaptive neural adaptation resulting from a greater reliance on the contralesional motor cortices.
Keywords: beta-band phase coherence; electroencephalography; electromyography; multimodal corticomuscular connectivity; phase coherence analysis; post-stroke neuroadaptation.
Conflict of interest statement
Conflict of Interest The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest. Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
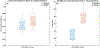

References
-
- Anwer S, Waris A, Gilani SO, Iqbal J, Shaikh N, Pujari AN and Niazi IK, 2022, January. Rehabilitation of upper limb motor impairment in stroke: A narrative review on the prevalence, risk factors, and economic statistics of stroke and state of the art therapies. Healthcare, 10 (2), pp. 190. - PMC - PubMed
-
- Bayraktaroglu Z, von Carlowitz-Ghori K, Curio G and Nikulin VV, 2013. It is not all about phase: amplitude dynamics in corticomuscular interactions. NeuroImage, 64, pp.496–504. - PubMed
-
- Boashash B, 1992. Estimating and interpreting the instantaneous frequency of a signal. I. Fundamentals. Proceedings of the IEEE, 80(4), pp.520–538.
Grants and funding
LinkOut - more resources
Full Text Sources
