Molecular mechanisms of sulforaphane in Alzheimer's disease: insights from an in-silico study
- PMID: 39493676
- PMCID: PMC11530583
- DOI: 10.1007/s40203-024-00267-4
Molecular mechanisms of sulforaphane in Alzheimer's disease: insights from an in-silico study
Abstract
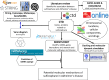
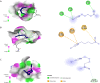
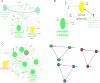
This study was to identify the molecular pathways that may explain sulforaphane's Alzheimer's disease (AD) benefits using multiple advanced in silico approaches. We found that sulforaphane regulates 45 targets, including TNF, INS, and BCL2. Therefore, it may help treat AD by reducing neuroinflammation, insulin resistance, and apoptosis. The important relationships were co-expression and pathways. 45 targets were linked to the midbrain, metabolite interconversion enzymes, 14q23.3 and 1q31.1 chromosomes, and modified residues. "Amyloid precursor protein catabolic process", "regulation of apoptotic signaling pathway", and "positive regulation of nitric oxide biosynthetic process" were the main pathways, while NFKB1, SP1, RELA, hsa-miR-17-5p, hsa-miR-16-5p, and hsa-miR-26b-5p were transcription factors and miRNAs implicated in sulforaphane In AD treatment, miRNA sponges, dexibuprofen, and sulforaphane may be effective. Furthermore, its unique physicochemical, pharmacokinetic, and biological qualities make sulforaphane an effective AD treatment, including efficient gastrointestinal absorption, drug-like properties, absence of CYP450 enzyme inhibition, not being a substrate for P-glycoprotein, ability to cross the blood-brain barrier, glutathione S-transferase substrate, immunostimulant effects, and antagonistic neurotransmitter effects. Sulforaphane is a promising compound for AD management. Further work is needed to elucidate its therapeutic effects based on our findings, including genes, miRNAs, molecular pathways, and transcription factors.
Supplementary information: The online version contains supplementary material available at 10.1007/s40203-024-00267-4.
Keywords: Alzheimer’s disease; In silico; Molecular mechanisms; Post-translational modification; Sulforaphane.
© The Author(s) 2024.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures




References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25
-
- Amaladoss N, Ramasamy V, Kuppusamy K (2024) Single crystal, conformational, quantum reactivity, hirshfeld surface, molecular interactions, ADMET, and molecular docking investigations on HIV-1 site of 3-isopropyldiphenyl-1-(2-(thiophen-2yl)acetyl)piperidin-4-one. J Mol Struct 1318:139352
-
- An YW et al (2016) Sulforaphane exerts its anti-inflammatory effect against amyloid-β peptide via STAT-1 dephosphorylation and activation of Nrf2/HO-1 cascade in human THP-1 macrophages. Neurobiol Aging 38:1–10. 10.1016/j.neurobiolaging.2015.10.016 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

