Serum and mucosal antibody-mediated protection and identification of asymptomatic respiratory syncytial virus infection in community-dwelling older adults in Europe
- PMID: 39493753
- PMCID: PMC11527605
- DOI: 10.3389/fimmu.2024.1448578
Serum and mucosal antibody-mediated protection and identification of asymptomatic respiratory syncytial virus infection in community-dwelling older adults in Europe
Abstract
Introduction: Respiratory syncytial virus (RSV) causes acute respiratory tract infection (ARTI) and reinfects adults throughout life, posing a risk for hospitalization in older adults (>60 years) with frailty and comorbidities.
Methods: To investigate serum and mucosal antibodies for protection against RSV infections, baseline serum samples were compared for RSV-pre- and -post-fusion (F) binding, and RSV-A2 neutralizing IgG antibodies between symptomatic RSV-ARTI (N = 30), non-RSV (RSV negative) ARTI (N = 386), and no ARTI (N = 338). Mucosal RSV-pre-F IgA and IgG levels, as well as serum RSV-G IgG antibodies, were analyzed to determine their association with protection from symptomatic RSV-ARTI in a subset study.
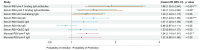
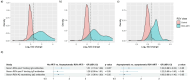
Results: Using a receiver operating characteristic (ROC) analysis, we established thresholds of 1.4- to 1.6-fold change (FC) for RSV-pre-F and -post-F, and RSV-A2 neutralizing IgG antibodies, respectively, enabling the identification of asymptomatic RSV cases with high sensitivity and specificity (>80% and >90%, respectively). As a result, serum RSV-pre-F, RSV-G IgG, and mucosal pre-F binding IgA antibodies showed correlations with protection against symptomatic RSV infection. RSV-pre-F IgG antibodies were correlated with protection from RSV infections irrespective of the symptoms.
Discussion: This study provides insights into antibody-mediated protection for symptomatic RSV infection in a community-dwelling older-adult population and establishes a threshold to identify asymptomatic RSV infection using a data-driven approach.
Keywords: RSV infections; humoral immunity; immune correlates; immune response; older adults; respiratory syncytial virus; symptomatic infections.
Copyright © 2024 Öner, Vernhes, Balla-Jhagjhoorsingh, Moureau, Crabbe, Salaun, Bastian, Thys, De Smedt, Ooft, Korsten, Adriaenssens, Coenen, Butler, Verheij, Drysdale, Wildenbeest, Pollard, Openshaw, Bont and Aerssens.
Conflict of interest statement
DÖ, SB-J, MC, AB, KT, SO, and JA were employed at the time the work was performed by Janssen Pharmaceuticals, a Johnson & Johnson company, and may be Johnson & Johnson stockholders. CV and AM were employed by Sanofi at the time the work was performed and may be Sanofi stockholder. CV is now employed by Vaccines Europe, Brussels, Belgium. BS and JD are employed by GlaxoSmithKline and may be GlaxoSmithKline stockholders. CB has participated in two virtual Advisory Boards for Moderna relevant to RSV vaccination on 30 March 2023 and 18 May 2023 and received payments. TV has received grant from Innovative Medicines Initiative European Commission, in which Biomerieux, Abbott, BD, BioRad, Janssen contributed ValueDx project, NIHR for project on cellulitis, and NHS UK regional funding for project on respiratory tract infections. He have been on the Advisory Board of a US study on lower respiratory tract infections in primary care EAST-PC funded by the Agency for Healthcare Research and Quality. He is a member of the Dutch Health Council. SD has provided consultancy and/or investigator roles in relation to product development for Janssen, AstraZeneca, Pfizer, Moderna, Valneva, MSD, iLiAD and Sanofi with fees paid to St George’s, University of London. SD has been on the RSV advisory board Sanofi Pasteur and received fees. SD also received support for travel for chairing a conference session, fees paid to his institution SGUL. SD is a member of the UK Department of Health and Social Care’s DHSC Joint Committee on Vaccination and Immunisation JCVI RSV subcommittee and Medicines and Healthcare products Regulatory Agency’s MHRA Paediatric Medicine Expert Advisory Group PMEAG. JW is investigator for clinical trials funded by pharmaceutical companies including AstraZeneca, Merck, Pfizer, Sanofi, and Janssen and investigator for clinical trials funded by IMI/Horizon2020 and ZonMw, with payments paid to her institution. She was a speaker at Sanofi sponsored symposium ESPID, payments paid to her institution. JW participated in the advisory board of Janssen RSV older adults and Sanofi advisory board with fees paid to UMCU. AP is part of EC IMI Programme RESCEU, payments paid to his institution. He has received grants from Gates, Wellcome, CEPI, MRC, NIHR, Serum Institute of India, AstraZeneca, and EC, with payments paid to his institution. Oxford University has entered into a partnership with AZ for development of COVID-19 vaccines. AP is a contributor to intellectual property licensed by Oxford University Innovation to AstraZeneca. AP is chair of the UK Department of Health and Social Care’s DHSC Joint Committee on Vaccination and Immunisation JCVI, NIHR Senior Investigator, member of the Academy of Medical Sciences AMS and was a member of WHO’s SAGE until January 2022. AP’s institution received funding from the European Commission’s IMI programme for the conduct of this study. Oxford University has entered a partnership with AstraZeneca for the development of COVID-19 vaccines. PO received honoraria GSK, Pfizer Inc, Sanofi Pasteur, Seqirus, Moderna and Janssen for participation in advisory boards and expert meetings and for acting as a speaker in congresses outside the scope of the submitted work. PO is also a principal investigator in the INFLAMMAGE trial co-funded by the Medical Research Council UK and GSK as part of the EMINENT consortium to promote inflammation research. He received grants from UKRI-MRC/DHSC NIHR Grant award MR/V027859/1, and UKRI-BEIS for human infection challenge with SARS-CoV-2 and had roles as school governor Sidcot, Somerset. LB has regular interaction with pharmaceutical and other industrial partners. He has not received personal fees or other personal benefits. UMCU has received major funding >€100,000 per industrial partner for investigator-initiated studies from AbbVie, MedImmune, AstraZeneca, Sanofi, Janssen, Pfizer, MSD and MeMed Diagnostics. UMCU has received major funding for the RSV GOLD study from the Bill and Melinda Gates Foundation. UMCU has received major funding as part of the public private partnership IMI-funded RESCEU and PROMISE projects with partners GSK, Novavax, Janssen, AstraZeneca, Pfizer and Sanofi. UMCU has received major funding from Julius Clinical for participating in clinical studies sponsored by MedImmune and Pfizer. UMCU received minor funding €1,000-25,000 per industrial partner for consultation and invited lectures by AbbVie, MedImmune, Ablynx, Bavaria Nordic, MabXience, GSK, Novavax, Pfizer, Moderna, AstraZeneca, MSD, Sanofi, Genzyme, Janssen. LB is the founding chairman of the ReSViNET Foundation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- CDC . Learn about RSV in older adults with chronic medical conditions, in: Centers for Disease Control and Prevention (2022). Available online at: https://www.cdc.gov/rsv/high-risk/older-adults.html (Accessed January 9, 2023).
-
- Shi T, Denouel A, Tietjen AK, Campbell I, Moran E, Li X, et al. Global disease burden estimates of respiratory syncytial virus–associated acute respiratory infection in older adults in 2015: A systematic review and meta-analysis. J Infect Dis. (2020) 222:S577–83. doi: 10.1093/infdis/jiz059 - DOI - PubMed
-
- Korsten K, Adriaenssens N, Coenen S, Butler C, Ravanfar B, Rutter H, et al. Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): an international prospective cohort study. Eur Respir J. (2021) 57:2002688. doi: 10.1183/13993003.02688-2020 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

