Device-Assisted Therapy in Non-Muscle-Invasive Bladder Cancer
- PMID: 39493816
- PMCID: PMC11530031
- DOI: 10.3233/BLC-240032
Device-Assisted Therapy in Non-Muscle-Invasive Bladder Cancer
Abstract
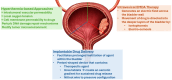
Intravesical therapy is a critical component in the management of non-muscle-invasive bladder cancer (NMIBC), as it reduces rates of disease recurrence and progression. However, the presence of physiologic barriers in the urothelium reduces the penetration and distribution of intravesical chemotherapy, thereby limiting the therapeutic potential. Much progress to overcome this challenge has been made in the realm of intravesical device-assisted therapy. Novel device-assisted treatments include hyperthermia, the radiofrequency-induced thermochemotherapy effect, electromotive drug administration, and implantable drug delivery systems. Notably, chemotherapy enhanced by these device-assisted systems has shown improved oncologic efficacy relative to standard intravesical chemotherapy and comparable outcomes relative to Bacillus Calmette-Guérin (BCG) therapy in patients with intermediate- or high-risk NMIBC. Recent studies also support the utility of device-assisted therapy as a salvage treatment option in patients with BCG-unresponsive disease. Ongoing randomized controlled trials and prospective investigations will further help clarify indications and long-term safety outcomes of these treatment modalities in NMIBC. Herein, we present a comprehensive review of device-assisted therapies and discuss their clinical utilities for the management of NMIBC in the modern era.
Keywords: Non-muscle-invasive bladder cancer; and drug delivery; electromotive drug administration; hyperthermia; intravesical chemotherapy; radiofrequency-induced thermochemotherapy.
© 2024 – The authors. Published by IOS Press.
Conflict of interest statement
S.G. and N.R. have no conflicts of interest to report.
Figures
References
-
- Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–5; discussion 475-7. doi: 10.1016/j.eururo.2005.12.031. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

