A Molecular Urine Assay to Detect Recurrences During Surveillance of High-Risk Non-Muscle Invasive Bladder Cancer
- PMID: 39493819
- PMCID: PMC11530022
- DOI: 10.3233/BLC-240017
A Molecular Urine Assay to Detect Recurrences During Surveillance of High-Risk Non-Muscle Invasive Bladder Cancer
Abstract
Background: High-risk non-muscle invasive bladder cancer (HR-NMIBC) patients require long-term surveillance with cystoscopies, cytology and upper tract imaging. Previously, we developed a genomic urine assay for surveillance of HR-NMIBC patients with high sensitivity and anticipatory value.
Objective: We aimed to validate the performance of the assay in an unselected prospectively collected cohort of HR-NMIBC patients under surveillance.
Methods: We included patients from five centers and collected urine sample pairs (evening and morning urines) prior to cystoscopy. Mutation status (FGFR3/TERT) and methylation status (OTX1) was analyzed on DNA from voided urine specimens. A test was considered positive if≥1 alteration was detected in at least one urine sample. The primary endpoint was tumor recurrence. Sensitivity and specificity were determined. A generalized mixed effects model was used to adjust for within-patient correlation. Cox proportional hazard analyses with time-dependent covariates assessed the anticipatory effect of the urine assay.
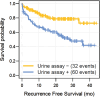
Results: In total, 204 patients and 736 sample pairs were collected. Sixty-three recurrences were diagnosed for which we had concomitant assay results. On cross-sectional analyses, the assay detected 75% (95% CI 62.1% -84.7%) of recurrences, with a specificity of 70% (95% CI 66.4% -73.5%). Furthermore, mixed effects model analyses revealed OTX1 (p = 0.005) and TERT (p = 0.004) as significant predictors for disease recurrence. Median follow-up was 25.3 months (IQR 18.6-30.7). Twenty-nine tumors were diagnosed without concomitant urine samples, which included recurrences detected after urine collection ended. Longitudinal analyses showed that a positive urine assay predicted a recurrence over time (HR 3.5, p < 0.001). Furthermore, a recurrence during the study period was also a predictor for developing future recurrences (HR 2.1, p < 0.001).
Conclusions: This study validates the performance of a previously developed urine assay in an unselected cohort of HR-NMIBC patients under surveillance. With a robust sensitivity/specificity and a strong anticipatory effect, this assay proves a useful adjunct ready for evaluation in a future randomized controlled trial.
Keywords: Keywords (MeSH): Bladder cancer; methylation; mutation; prospective; urinary biomarkers.
© 2024 – The authors. Published by IOS Press.
Conflict of interest statement
TZ and EZ are Editorial Board members of this journal, but were not involved in the peer-review process nor had access to any information regarding its peer-review. TZ declares Grant/Research support from: Genecentric/Janssen, InnoSign and Merk AG (all paid to Erasmus MC). EZ declares a License agreement with MDxHealth / Vesica and a patent for OTX1 and ONECUT2 methylation issued in EU and Canada, and a pending patent in the USA. JB declares consultantcy / speaker’s fee (all paid to Erasmus MC) from: Janssen, BMS, AstraZeneca, Merck AG/Pfizer, MSD, Bayer, Ismar Healtcare. Research collaborations with: Merck AG, MSD, Janssen, VitroScan and Merk Serono. Member of the Scientific Congress Committee European Association of Urology. JJ, FJ, AM, NC, HR, EO, RP, EW and CB declare no conflicts of interest related to this topic nor to the contents of this manuscript.
Figures
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. - PubMed
-
- Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al.. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022;81(1):75–94. - PubMed
-
- van der Aa MN, Steyerberg EW, Bangma C, van Rhijn BW, Zwarthoff EC, van der Kwast TH. Cystoscopy revisited as the gold standard for detecting bladder cancer recurrence: diagnostic review bias in the randomized, prospective CEFUB trial. The Journal of Urology. 2010;183(1):76–80. - PubMed
-
- Beukers W, van der Keur KA, Kandimalla R, Vergouwe Y, Steyerberg EW, Boormans JL, et al.. FGFR3, TERT and OTX1 as a Urinary Biomarker Combination for Surveillance of Patients with Bladder Cancer in a Large Prospective Multicenter Study. J Urol. 2017;197(6):1410–8. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous