Single high-fat challenge and trained innate immunity: A randomized controlled cross-over trial
- PMID: 39493874
- PMCID: PMC11530819
- DOI: 10.1016/j.isci.2024.111103
Single high-fat challenge and trained innate immunity: A randomized controlled cross-over trial
Abstract
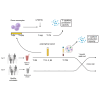
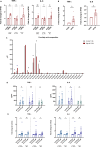
Brief exposure of monocytes to atherogenic molecules, such as oxidized lipoproteins, triggers a persistent pro-inflammatory phenotype, named trained immunity. In mice, transient high-fat diet leads to trained immunity, which aggravates atherogenesis. We hypothesized that a single high-fat challenge in humans induces trained immunity. In a randomized controlled cross-over study, 14 healthy individuals received a high-fat or reference shake, and blood was drawn before and after 1, 2, 4, 6, 24, and 72 h. Incubation of donor monocytes with the post-high-fat-shake serum induced trained immunity, regulated via Toll-like receptor 4. This was not mediated via triglyceride-rich lipoproteins, C12, 14, and 16, or metabolic endotoxemia. In vivo, however, the high-fat challenge did not affect monocyte phenotype and function. We conclude that a high-fat challenge leads to alterations in the serum composition that have the potential to induce trained immunity in vitro. However, this does not translate into a (persistent) hyperinflammatory monocyte phenotype in vivo.
Keywords: Health sciences; Human Physiology; Human metabolism.
© 2024 The Author(s).
Conflict of interest statement
M.G.N. and L.A.B.J. are scientific founders of TTxD and Lemba Therapeutics.
Figures



References
-
- GBD 2017 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/S0140-6736(18)32335-3. - DOI - PMC - PubMed
-
- Gower R.M., Wu H., Foster G.A., Devaraj S., Jialal I., Ballantyne C.M., Knowlton A.A., Simon S.I. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler. Thromb. Vasc. Biol. 2011;31:160–166. doi: 10.1161/ATVBAHA.110.215434. - DOI - PMC - PubMed
-
- Esser D., Oosterink E., op 't Roodt J., Henry R.M.A., Stehouwer C.D.A., Müller M., Afman L.A. Vascular and inflammatory high fat meal responses in young healthy men; a discriminative role of IL-8 observed in a randomized trial. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053474. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

