Polyurethane Waste Recycling: Thermolysis of the Carbamate Fraction
- PMID: 39494010
- PMCID: PMC11525496
- DOI: 10.1021/acsomega.4c04671
Polyurethane Waste Recycling: Thermolysis of the Carbamate Fraction
Abstract
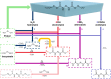
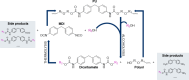
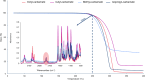
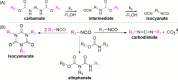
The alcoholysis of polyurethane waste is currently being industrialized, making it one of the most advanced chemical recycling processes for polyurethanes. However, the recycling potential of the dicarbamate phase, which accounts for 10-40% of the polyurethane mass, is often disregarded, as mainly the polyol components are (partially) retrieved in many alcoholysis processes. In this study, we present a two-step recycling method in which the valuable carbamate fraction obtained in the initial alcoholysis step is transformed into an isocyanate-rich mixture through an additional thermolysis step. For this purpose, different carbamates were synthesized and thermolyzed, which showed that thermolysis with isopropyl-carbamate was the most favorable, obtaining a yield of 35%. As a result, the isocyanates obtained through thermolysis and the polyols obtained through alcoholysis can be reused as starting materials in polyurethane synthesis.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- ″in Europe, Middle East and Africa (EMEA) - 16th ed. (including Raw Materials Vol.),″ 2023, doi: 10.1016/0012-1606(69)90033-5. - DOI
-
- Fonseca L. P.; et al. Reducing the carbon footprint of polyurethanes by chemical and biological depolymerization: Fact or fiction?. Curr. Opin. Green Sustainable Chem. 2023, 41, 100802 10.1016/j.cogsc.2023.100802. - DOI
-
- Rossignolo G.; Malucelli G.; Lorenzetti A. Recycling of polyurethanes: where we are and where we are going. Green Chem. 2024, 26 (3), 1132–1152. 10.1039/D3GC02091F. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
