NEXAFS Spectra Simulations of Nitrogen-Bearing Heterocycles
- PMID: 39494021
- PMCID: PMC11525529
- DOI: 10.1021/acsomega.4c07024
NEXAFS Spectra Simulations of Nitrogen-Bearing Heterocycles
Abstract
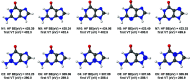
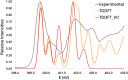
Five-membered heterocyclic compounds containing nitrogen atoms are important biomolecule building blocks. In addition to their fundamental biological importance, these molecular structures are used in several technological applications. Consequently, it is essential to develop techniques that allow the characterization of these fundamental systems. We address this issue by performing simulations of K-edge NEXAFS spectra by applying a time-dependent density functional theory (TDDFT) and an inner-shell multiconfigurational self-consistent field (IS-MCSCF) of selected molecules. Also, vibronic coupling simulations were considered for the TDDFT computations. Surprisingly, molecular orbital binding energies do not reproduce the order of the transition energies obtained by IS-MCSCF, indicating a possible breakdown of the orbital picture concerning the NEXAFS spectrum. In general, the TDDFT and IS-MCSCF results are compatible and are in close agreement with experimental data. Moreover, vibronic coupling and vertical transition results were very similar. Finally, it is important to mention that, to the best of our knowledge, this is the first time that the IS-MCSCF method has been applied to molecular systems of this size.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

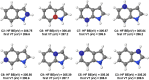

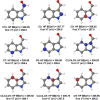

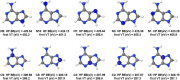
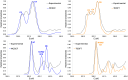

References
-
- Walsh C. T. Nature loves nitrogen heterocycles. Tetrahedron Lett. 2015, 56, 3075–3081. 10.1016/j.tetlet.2014.11.046. - DOI
-
Memorial Symposium-in-Print for Harry Wasserman
-
- Mendes J. O.; da Silva E. C.; Rocha A. B. On the nature of inhibition performance of imidazole on iron surface. Corros. Sci. 2012, 57, 254–259. 10.1016/j.corsci.2011.12.011. - DOI
-
- Kokalj A. Formation and structure of inhibitive molecular film of imidazole on iron surface. Corros. Sci. 2013, 68, 195–203. 10.1016/j.corsci.2012.11.015. - DOI
LinkOut - more resources
Full Text Sources
