Tailored biosynthesis of diosmin through reconstitution of the flavonoid pathway in Nicotiana benthamiana
- PMID: 39494057
- PMCID: PMC11527692
- DOI: 10.3389/fpls.2024.1464877
Tailored biosynthesis of diosmin through reconstitution of the flavonoid pathway in Nicotiana benthamiana
Abstract
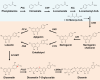
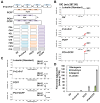
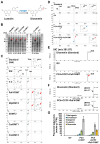
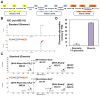
The flavonoid diosmin (diosmetin 7-O-rutinoside) is used as a therapeutic agent for disorders of the blood vessels such as hemorrhoids and varicose veins. Diosmin is commercially produced using semi-synthetic methods involving the oxidation of hesperidin, the most abundant flavonoid in citrus fruits. However, this method produces byproducts that are toxic to the environment, and new sustainable methods to produce diosmin are required. Here, we used a synthetic biology approach to produce diosmin without generating toxic byproducts through reconstitution of the diosmin biosynthetic pathway in Nicotiana benthamiana. We first established that N. benthamiana leaves co-infiltrated with all seven genes in the flavonoid biosynthesis pathway produced high levels of luteolin, a precursor of diosmetin. We then compared the activity of modification enzymes such as methyltransferases, glucosyltransferases, and rhamnosyltransferases in Escherichia coli and in planta and selected genes encoding enzymes with the highest activity for producing diosmetin, diosmetin 7-O-glucoside, and diosmin, respectively. Finally, we reconstructed the entire diosmin biosynthetic pathway using three constructs containing ten genes encoding enzymes in this pathway, from phenylalanine ammonia lyase to rhamnosyltransferase. N. benthamiana leaves transiently co-expressing all these genes yielded 37.7 µg diosmin per gram fresh weight. To our knowledge, this is the first report of diosmin production in a heterologous plant system without the supply of a precursor. Successful production of diosmin in N. benthamiana opens new avenues for producing other commercially important flavonoids using similar platforms.
Keywords: Nicotiana benthamiana; diosmetin; diosmin; flavonoid; synthetic biology; transient expression.
Copyright © 2024 Lee, Park, Lee, Song, Kim and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Abdel-Reheim M. A., Messiha B. A. S., Abo-Saif A. A. (2017). Hepatoprotective effect of diosmin on iron-induced liver damage. Int. J. Pharmacol. 13, 529–540. doi: 10.3923/ijp.2017.529.540 - DOI
-
- Ahmed S., Mundhe N., Borgohain M., Chowdhury L., Kwatra M., Bolshette N., et al. (2016). Diosmin modulates the NF-kB signal transduction pathways and downregulation of various oxidative stress markers in alloxan-induced diabetic nephropathy. Inflammation 39, 1783–1797. doi: 10.1007/s10753-016-0413-4 - DOI - PubMed
LinkOut - more resources
Full Text Sources

