Direct delivery of Cas9 or base editor protein and guide RNA complex enables genome editing in the retina
- PMID: 39494148
- PMCID: PMC11531619
- DOI: 10.1016/j.omtn.2024.102349
Direct delivery of Cas9 or base editor protein and guide RNA complex enables genome editing in the retina
Abstract
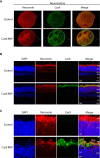
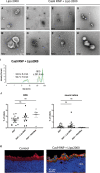
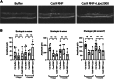
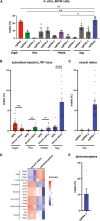
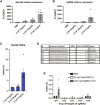
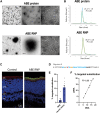
Genome editing by CRISPR-Cas holds promise for the treatment of retinal dystrophies. For therapeutic gene editing, transient delivery of CRISPR-Cas9 is preferable to viral delivery which leads to long-term expression with potential adverse consequences. Cas9 protein and its guide RNA, delivered as ribonucleoprotein (RNP) complexes, have been successfully delivered into the retinal pigment epithelium in vivo. However, the delivery into photoreceptors, the primary focus in retinal dystrophies, has not been achieved. Here, we investigate the feasibility of direct RNP delivery into photoreceptors and retinal pigment epithelium cells. We demonstrate that Cas9 or adenine-base editors complexed with guide RNA, can enter retinal cells without the addition of any carrier compounds. Once in the retinal cells, editing rates vary based on the efficacy of the guide RNA and the specific location edited within the genes. Cas9 RNP delivery at high concentrations, however, leads to outer retinal toxicity. This underscores the importance of improving delivery efficiency for potential therapeutic applications in the future.
Keywords: CRISPR/Cas9; MT: Delivery Strategies; RPE; base editor; gene editing; gene therapy; inherited retinal dystrophy; photoreceptor; retina.
© 2024 The Author(s).
Conflict of interest statement
D.D. is a co-inventor on patent #9193956 (Adeno-associated virus virions with variant capsid and methods of use thereof), with royalties paid to Adverum Biotechnologies and on pending patent applications on noninvasive methods to target cone photoreceptors (EP17306429.6 and EP17306430.4) licensed to Gamut Tx now SparingVision. D.D. also has personal financial interests in Tenpoint Tx. and SparingVision, outside the scope of the submitted work.
Figures

References
-
- Maguire A.M., Russell S., Chung D.C., Yu Z.-F., Tillman A., Drack A.V., Simonelli F., Leroy B.P., Reape K.Z., High K.A., Bennett J. Durability of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease: Phase 3 Results at 3 and 4 Years. Ophthalmology. 2021;128:1460–1468. doi: 10.1016/j.ophtha.2021.03.031. - DOI - PubMed
-
- Fischer M.D., Michalakis S., Wilhelm B., Zobor D., Muehlfriedel R., Kohl S., Weisschuh N., Ochakovski G.A., Klein R., Schoen C., et al. Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia. JAMA Ophthalmol. 2020;138:1–9. doi: 10.1001/jamaophthalmol.2020.1032. - DOI - PMC - PubMed
-
- Xue K., Jolly J.K., Barnard A.R., Rudenko A., Salvetti A.P., Patrício M.I., Edwards T.L., Groppe M., Orlans H.O., Tolmachova T., et al. Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat. Med. 2018;24:1507–1512. doi: 10.1038/s41591-018-0185-5. - DOI - PMC - PubMed
-
- Cukras C., Wiley H.E., Jeffrey B.G., Sen H.N., Turriff A., Zeng Y., Vijayasarathy C., Marangoni D., Ziccardi L., Kjellstrom S., et al. Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery. Mol. Ther. 2018;26:2282–2294. doi: 10.1016/j.ymthe.2018.05.025. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

