Cipaglucosidase alfa plus miglustat: linking mechanism of action to clinical outcomes in late-onset Pompe disease
- PMID: 39494167
- PMCID: PMC11527667
- DOI: 10.3389/fneur.2024.1451512
Cipaglucosidase alfa plus miglustat: linking mechanism of action to clinical outcomes in late-onset Pompe disease
Erratum in
-
Corrigendum: Cipaglucosidase alfa plus miglustat: linking mechanism of action to clinical outcomes in late-onset Pompe disease.Front Neurol. 2025 Jan 3;15:1540452. doi: 10.3389/fneur.2024.1540452. eCollection 2024. Front Neurol. 2025. PMID: 39830206 Free PMC article.
Abstract
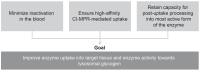
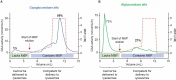
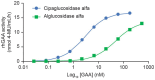
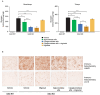
Enzyme replacement therapy (ERT) is the only approved disease-modifying treatment modality for Pompe disease, a rare, inherited metabolic disorder caused by a deficiency in the acid α-glucosidase (GAA) enzyme that catabolizes lysosomal glycogen. First-generation recombinant human GAA (rhGAA) ERT (alglucosidase alfa) can slow the progressive muscle degeneration characteristic of the disease. Still, most patients experience diminished efficacy over time, possibly because of poor uptake into target tissues. Next-generation ERTs aim to address this problem by increasing bis-phosphorylated high mannose (bis-M6P) N-glycans on rhGAA as these moieties have sufficiently high receptor binding affinity at the resultant low interstitial enzyme concentrations after dosing to drive uptake by the cation-independent mannose 6-phosphate receptor on target cells. However, some approaches introduce bis-M6P onto rhGAA via non-natural linkages that cannot be hydrolyzed by natural human enzymes and thus inhibit the endolysosomal glycan trimming necessary for complete enzyme activation after cell uptake. Furthermore, all rhGAA ERTs face potential inactivation during intravenous delivery (and subsequent non-productive clearance) as GAA is an acid hydrolase that is rapidly denatured in the near-neutral pH of the blood. One new therapy, cipaglucosidase alfa plus miglustat, is hypothesized to address these challenges by combining an enzyme enriched with naturally occurring bis-M6P N-glycans with a small-molecule stabilizer. Here, we investigate this hypothesis by analyzing published and new data related to the mechanism of action of the enzyme and stabilizer molecule. Based on an extensive collection of in vitro, preclinical, and clinical data, we conclude that cipaglucosidase alfa plus miglustat successfully addresses each of these challenges to offer meaningful advantages in terms of pharmacokinetic exposure, target-cell uptake, endolysosomal processing, and clinical benefit.
Keywords: Pompe disease; enzyme replacement therapy; glycogen storage disease type II; lysosomal storage disorders; n-butyldeoxynojirimycin.
Copyright © 2024 Byrne, Parenti, Schoser, van der Ploeg, Do, Fox, Goldman, Johnson, Kang, Mehta, Mondick, Sheikh, Sitaraman Das, Tuske, Brudvig, Weimer and Mozaffar.
Conflict of interest statement
BB reports consultant/advisory board membership for Pfizer, Amicus Therapeutics, Inc., and Sanofi; and owns stocks in Lacerta Therapeutics. GP received honoraria, travel reimbursement and research support from Sanofi, Takeda, Piam Farmaceutici, and Spark Therapeutics. BS has received unrestricted research grants from AMDA Foundation, Amicus Therapeutics, Inc., EU Horizon programs COMPASS and PaLaDIn, Marigold Foundation, Roche Diagnostics, and speaker’s honoraria from Alexion, Amicus Therapeutics, Inc., Argenx, Kedrion, and Sanofi. He has also been a scientific advisor for Amicus Therapeutics, Inc., Alexion, Astellas, Sanofi, and Taysha. He declares no stocks or shares. AP is an advisory board member of Amicus Therapeutics, Inc., BioMarin, Sanofi Genzyme, and Spark Therapeutics. She provided consultancies for Amicus Therapeutics, Inc., BioMarin, Sanofi Genzyme, and Spark Therapeutics; and contracted research for Amicus Therapeutics, Inc., BioMarin, Sanofi Genzyme, and Spark Therapeutics. All collaborations were carried out under an agreement between Erasmus MC and these industries. HD is a former employee of Amicus Therapeutics, Inc. and a current employee of 6MP-Therapeutics. BF, MG, FJ, NM, OS, SS, ST, JB, and JW are current employees of and holds stock in Amicus Therapeutics, Inc. JK is an employee of Metrum Research Group, which was contracted by Amicus to perform the PK/PD analysis, and has no other competing interests to declare. JM was a paid consultant as an employee of Metrum Research Group when the modeling work was carried out JM was employed by Incyte Corporation at the time the manuscript was developed.TM has advised for Abbvie, Alexion, Amicus Therapeutics, Inc., Annji, Argenx, Arvinas, Audentes, Cabaletta, Maze Therapeutics, Momenta, Ra Pharmaceuticals, Sanofi Genzyme, Sarepta, Spark Therapeutics, and UCB. He participates on the speaker’s bureau for Sanofi Genzyme and the medical advisory boards for the Myositis Association, Neuromuscular Disease Foundation, Myasthenia Gravis Foundation of California and Myasthenia Gravis Foundation of America. He has received research funding from the Myositis Association, the Muscular Dystrophy Association, the NIH and from the following sponsors: Alexion, Amicus Therapeutics, Inc., Annji, Argenx, Audentes, Bristol-Myers Squibb, Cabaletta, Cartesian Therapeutics, Grifols, Momenta, Ra Pharmaceuticals, Sanofi Genzyme, Spark Therapeutics, UCB, and Valerion; and is on the data safety monitoring board for Acceleron, Applied Therapeutics, Sarepta, and the NIH. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures







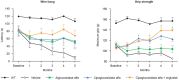


References
-
- Reuser AJJ, Hirschhorn R, Kroos MA. Pompe disease: glycogen storage disease type II, acid α-glucosidase (acid maltase) deficiency In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA, editors. The online metabolic and molecular bases of inherited disease. New York, NY: McGraw-Hill Education; (2019)
-
- Cabello JF, Marsden D. Pompe disease: clinical perspectives. Orphan Drugs Res Rev. (2017) 7:1–10. doi: 10.2147/ODRR.S69109 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

