The Mechanism by Which Cyperus rotundus Ameliorates Osteoarthritis: A Work Based on Network Pharmacology
- PMID: 39494203
- PMCID: PMC11531273
- DOI: 10.2147/JIR.S483652
The Mechanism by Which Cyperus rotundus Ameliorates Osteoarthritis: A Work Based on Network Pharmacology
Abstract
Background: Cyperus rotundus (CR) is widely used in traditional Chinese medicine to prevent and treat a variety of diseases. However, its functions and mechanism of action in osteoarthritis (OA) has not been elucidated. Here, a comprehensive strategy combining network pharmacology, molecular docking, molecular dynamics simulation and in vitro experiments was used to address this issue.
Methods: The bioactive ingredients of CR were screened in TCMSP database, and the potential targets of these ingredients were obtained through Swiss Target Prediction database. Genes in OA pathogenesis were collected through GeneCards, OMIM and DisGeNET databases. Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were performed using DAVID database. STRING database and Cytoscape 3.10 software were used to construct "component-target-pathway" network, and predict the core targets affected by CR. The binding affinity between bioactive components and the core targets was evaluated by molecular docking and molecular dynamics simulation. The therapeutic activity of kaempferol on chondrocytes in inflammatory conditions was verified by in vitro experiments.
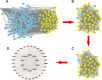
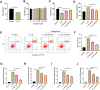
Results: Fifteen CR bioactive ingredients were obtained, targeting 192 OA-related genes. A series of biological processes, cell components, molecular functions and pathways were predicted to be modulated by CR components. The core targets of CR in OA treatment were AKT serine/threonine kinase 1 (AKT1), interleukin 1 beta (IL1B), SRC proto-oncogene, non-receptor tyrosine kinase (SRC), BCL2 apoptosis regulator (BCL2), signal transducer and activator of transcription 3 (STAT3), epidermal growth factor receptor (EGFR), hypoxia-inducible factor 1 subunit alpha (HIF1A), matrix metallopeptidase 9 (MMP9), estrogen receptor 1 (ESR1) and PPARG orthologs from vertebrates (PPARG), and the main bioactive ingredients of CR showed good binding affinity with these targets. In addition, kaempferol, one of the CR bioactive components, weakens the effects of IL-1β on the viability, apoptosis and inflammation of chondrocytes.
Conclusion: Theoretically, CR has great potential to ameliorate the symptoms and progression of OA, via multiple components, multiple targets, and multiple downstream pathways.
Keywords: Cyperus rotundus; molecular docking; network pharmacology; osteoarthritis.
© 2024 Du et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

