N-Acetylcysteine Alleviates Depressive-Like Behaviors in Adolescent EAAC1-/- Mice and Early Life Stress Model Rats
- PMID: 39494328
- PMCID: PMC11528454
- DOI: 10.7150/ijbs.97723
N-Acetylcysteine Alleviates Depressive-Like Behaviors in Adolescent EAAC1-/- Mice and Early Life Stress Model Rats
Abstract
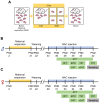
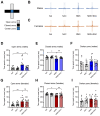
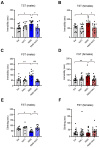
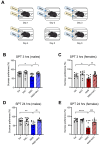
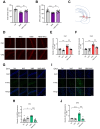
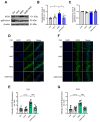
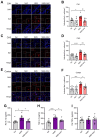
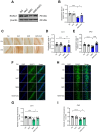
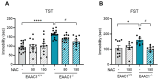
Exposure to adverse experiences during early life is associated with an increased risk of psychopathology during adolescence. In a previous study, we demonstrated that neonatal maternal separation (NMS) combined with social isolation led to impulsive and depressive-like behaviors in male adolescents. Additionally, it significantly reduced the expression of excitatory amino acid carrier 1 (EAAC1) in the hippocampus. Building upon this work, we investigated the effects of N-acetylcysteine (NAC), a precursor to glutathione, in early-life stress (ELS) model rats and in EAAC1-/- mice. EAAC1 plays a dual role in transporting both glutamate and cysteine into neurons. Our findings revealed that female adolescents subjected to in the ELS model also exhibited behavioral defects similar to those of males. NAC injection rescued depressive-like behaviors in both male and female NMS models, but it improved impulsive behavior only in males. Furthermore, we observed increased reactive oxidative stress (ROS) and neuroinflammation in the ventral hippocampus (vHPC) and prefrontal cortex of NMS model rats, which were mitigated by NAC treatment. Notably, NAC reversed the reduced expression of EAAC1 in the vHPC of NMS model rats. In EAAC1-/- mice, severe impulsive and depressive-like behaviors were evident, and the NAC intervention improved only depressive-like behaviors. Collectively, our results suggest that ELS contributes to depression and impulsive behaviors during adolescence. Moreover, the cysteine uptake function of EAAC1 in neurons may be specifically related to depression rather than impulsive behavior.
Keywords: Depressive-like behavior; Early life stress; Excitatory amino acid carrier 1 (EAAC1); Impulsive behavior; N-acetylcysteine (NAC); Neonatal maternal separation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Harris T, Brown GW, Bifulco A. Loss of parent in childhood and adult psychiatric disorder: the role of lack of adequate parental care. Psychol Med. 1986;16(3):641–59. - PubMed
-
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H. et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4(2):163–72. - PubMed
-
- Loeber R, Stouthamer-Loeber M. Development of juvenile aggression and violence. Some common misconceptions and controversies. Am Psychol. 1998;53(2):242–59. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

